
Introduction
Enteric fever is a systemic infectious disease primarily affecting the gastrointestinal tract and reticuloendothelial system.
-
It is caused by:
-
Salmonella enterica serovar Typhi (Typhoid fever)
-
Salmonella enterica serovar Paratyphi (Paratyphoid fever)
-
-
The disease is transmitted through the fecal–oral route, mainly by ingestion of:
-
Contaminated food
-
Contaminated water
-
-
It is closely associated with:
-
Poor sanitation
-
Unsafe drinking water
-
Inadequate sewage disposal
-
Poor personal hygiene
-
-
Enteric fever is more common in:
-
Developing countries
-
Overcrowded communities
-
Areas with limited access to clean water
-
-
After ingestion, the organism:
-
Invades intestinal mucosa
-
Multiplies in Peyer’s patches
-
Enters bloodstream (bacteremia)
-
Spreads to liver, spleen, and bone marrow
-
-
Clinically characterized by:
-
Prolonged high-grade fever
-
Headache
-
Abdominal pain
-
Weakness
-
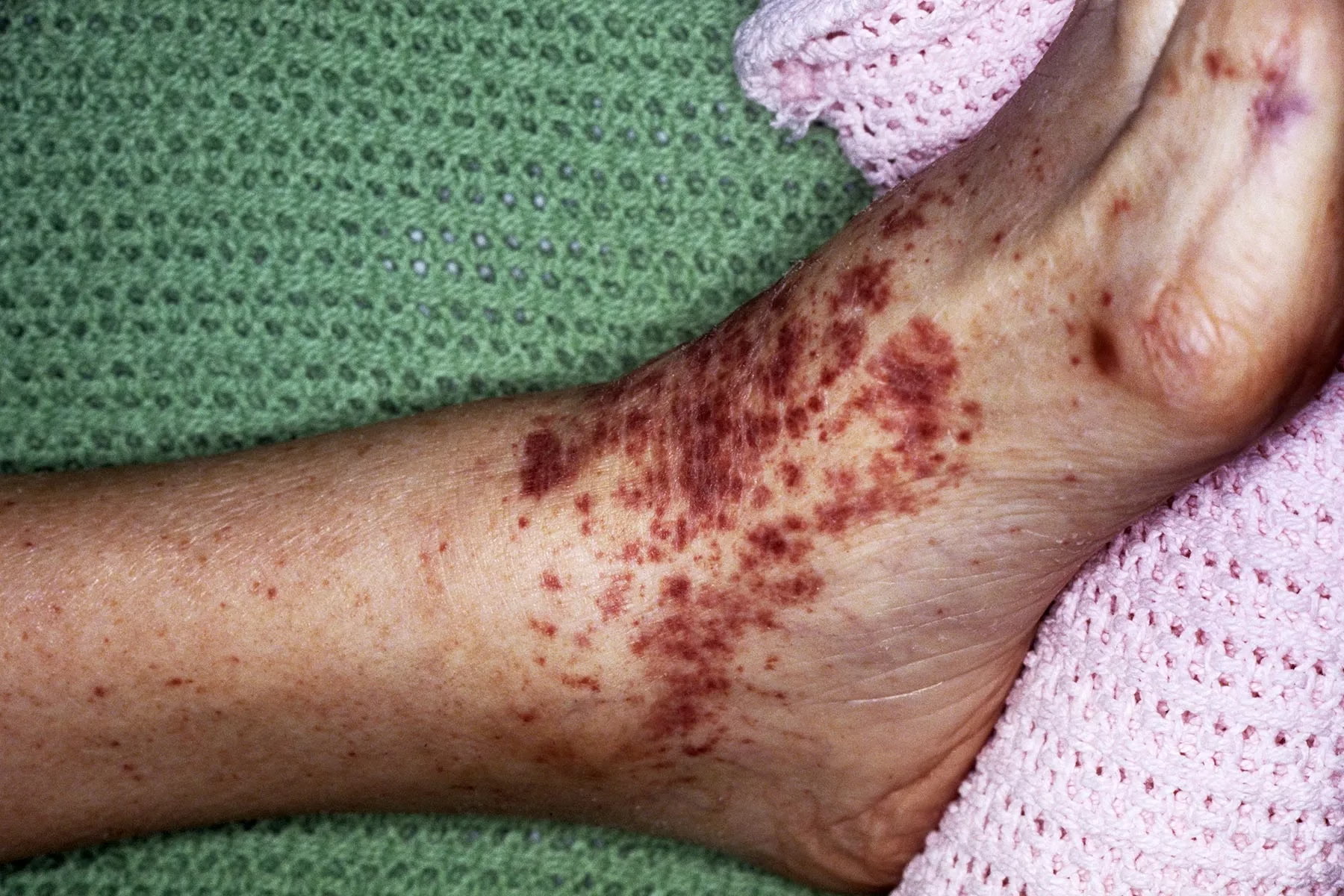
Rose spots (in some patients)
-
Etiological Agent
-
Enteric fever is caused by Gram-negative bacilli belonging to the family Enterobacteriaceae.
-
The main causative organisms are:
-
Salmonella enterica serovar Typhi
-
Salmonella enterica serovar Paratyphi (A, B, C)
-
-
S. Typhi causes classical and more severe typhoid fever.
-
S. Paratyphi usually causes a milder form of the disease.
Morphological Characteristics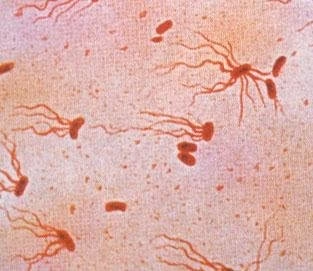
-
Gram-negative rods
-
Motile (peritrichous flagella)
-
Non-spore forming
-
Facultative anaerobes
Important Antigens
-
O antigen – Somatic antigen (heat stable)
-
H antigen – Flagellar antigen (heat labile)
-
Vi antigen – Capsular antigen (important virulence factor in S. Typhi)
Virulence Factors
-
Ability to survive within macrophages
-
Endotoxin (LPS) causing systemic symptoms
-
Vi antigen helps in immune evasion
Epidemiology
-
Enteric fever is a waterborne and foodborne disease.


-
It is endemic in:
-
South Asia
-
Southeast Asia
-
Sub-Saharan Africa
-
Parts of Latin America
-
-
High incidence in countries with:
-
Poor sanitation
-
Inadequate sewage disposal
-
Unsafe drinking water
-
-
Commonly affects:
-
Children and young adults
-
Low socioeconomic populations
-
-
Transmission occurs through:
-
Contaminated water
-
Contaminated food
-
Food handlers who are chronic carriers
-
-
Chronic carriers harbor bacteria in the gallbladder and shed organisms in stool.
-
Classic example: Mary Mallon
-
-
Incidence increases during:
-
Rainy seasons
-
Floods
-
Natural disasters
-
-
Global burden remains significant despite availability of vaccines.
Mode of Transmission

-
Enteric fever is transmitted mainly by the fecal–oral route.
-
Infection occurs through ingestion of:
-
Contaminated drinking water
-
Contaminated food
-
Raw vegetables washed with unsafe water
-
Street food prepared under unhygienic conditions
-
-
Sources of infection:
-
Patients in acute stage
-
Chronic carriers (organism persists in gallbladder)
-
-
Chronic carrier example:
-
Mary Mallon
-
-
Flies may act as mechanical vectors, carrying organisms from feces to food.
-
Person-to-person transmission may occur in areas with poor hygiene.
Pathogenesis
-
Ingestion of contaminated food/water.
-
Organisms survive gastric acid (especially when gastric acidity is low).
-
Reach small intestine → invade Peyer’s patches (lymphoid tissue of ileum).
-
Taken up by macrophages and multiply intracellularly.
-
Enter bloodstream → Primary bacteremia (often asymptomatic).
-
Localize in reticuloendothelial system:
-
Liver
-
Spleen
-
Bone marrow
-
-
Multiply further → Secondary bacteremia → Clinical symptoms appear.
-
Reinvasion of intestine → Inflammation, necrosis, and ulceration of Peyer’s patches.
-
Severe cases may lead to intestinal hemorrhage or perforation.
Clinical Features
-
Incubation period: 7–14 days (range 3–60 days).
General Symptoms
-
Prolonged high-grade fever (step-ladder pattern)
-
Headache
-
Malaise and weakness
-
Loss of appetite
-
Body ache
Gastrointestinal Symptoms
-
Abdominal pain
-
Constipation (early stage)
-
Diarrhea (later stage)
-
Coated tongue
Physical Signs
-
Relative bradycardia (Faget sign)
-
Hepatosplenomegaly
-
Abdominal tenderness
Characteristic Sign
-
Rose spots:
-
Faint salmon-colored maculopapular rash
-
Seen on trunk
-
Usually appears in 2nd week
-
Severe Cases
-
Delirium (“Typhoid state”)
-
Encephalopathy
Complications
Intestinal Complications
-
Intestinal hemorrhage
-
Intestinal perforation (most serious)
-
Peritonitis
Systemic Complications
-
Septic shock
-
Encephalopathy
-
Myocarditis
-
Pneumonia
-
Hepatitis
Chronic Complication
-
Chronic carrier state
Mortality increases significantly if complications are not managed promptly.
Laboratory Diagnosis
A. Specimen Collection (According to Week of Illness)
-
1st week → Blood culture (best method)
-
2nd week → Widal test, blood culture
-
3rd week onward → Stool and urine culture
-
Bone marrow → Any stage (highest sensitivity)
B. Direct Detection of Organism (Culture Methods)
1. Blood Culture (Gold Standard in 1st Week)
-
Most reliable early diagnostic method
-
5–10 mL blood collected before antibiotics
-
Culture media:
-
Bile broth
-
Automated blood culture systems
-
-
Positive in 60–80% cases (early stage)
Advantages:
-
Confirms diagnosis
-
Allows antibiotic sensitivity testing
2. Bone Marrow Culture
-
Most sensitive method (up to 90–95%)
-
Remains positive even after antibiotic therapy
-
Useful in complicated or treated cases
3. Stool Culture
-
Positive from 2nd–3rd week
-
Useful for detecting carriers
-
Selective media:
-
MacConkey agar
-
XLD agar
-
DCA agar
-
4. Urine Culture
-
May be positive in later stages
-
Less sensitive than blood culture
C. Identification of Isolate
-
Gram-negative bacilli on Gram staining
-
Biochemical tests:
-
Non-lactose fermenter
-
TSI: Alkaline slant / Acid butt with H₂S
-
-
Serotyping with O, H, and Vi antisera
Causative organisms:
-
Salmonella enterica serovar Typhi
-
Salmonella enterica serovar Paratyphi
D. Serological Tests
1. Widal Test
-
Detects antibodies against:
-
O antigen
-
H antigen
-
-
Becomes positive after 7–10 days
Interpretation:
-
Significant rise in antibody titer
-
Single high titer in endemic areas may be misleading
Limitations:
-
False positives
-
False negatives
-
Baseline titers vary in endemic regions
2. Rapid Tests
-
TyphiDot
-
IgM/IgG ELISA
-
Tubex test
Advantages:
-
Quick results
-
Useful in resource-limited settings
Limitations:
-
Variable sensitivity and specificity
E. Molecular Methods
-
PCR (Polymerase Chain Reaction)
-
High sensitivity
-
Useful in early diagnosis
-
Expensive and not widely available
F. Hematological Findings (Supportive)
-
Leukopenia
-
Relative lymphocytosis
-
Mild anemia
-
Elevated ESR
-
Thrombocytopenia (in severe cases)
Prevention & Control
A. Personal Preventive Measures
-
Drink safe and treated water (boiled, filtered, chlorinated).
-
Avoid consumption of:
-
Uncovered street food
-
Raw vegetables washed with unsafe water
-
-
Practice proper hand hygiene:
-
Before eating
-
After using toilet
-
-
Maintain proper food hygiene:
-
Thorough cooking
-
Safe storage
-
-
Avoid close contact with infected individuals during acute illness.
B. Community-Level Control Measures
-
Provision of safe drinking water supply.
-
Proper sewage disposal systems.
-
Improvement of sanitation facilities.
-
Health education regarding hygiene practices.
-
Regular inspection of food establishments.
-
Surveillance during outbreaks.
C. Identification and Management of Carriers
-
Chronic carriers may harbor organisms in the gallbladder.
-
Regular stool examination in food handlers.
-
Appropriate antibiotic therapy for carriers.
-
In some cases, cholecystectomy may be required.
D. Vaccination
Vaccination plays a key role in prevention, especially in endemic areas.
Available Vaccines:
-
Vi Polysaccharide Vaccine
-
Injectable
-
Given after 2 years of age
-
Booster every 3 years
-
-
Ty21a Vaccine
-
Live attenuated oral vaccine
-
Given in capsule form
-
-
Typhoid Conjugate Vaccine (TCV)
-
Can be given from 6 months of age
-
Provides longer immunity
-
Recommended in national immunization programs of endemic countries
-
E. Public Health Strategies
-
Early diagnosis and prompt treatment.
-
Antibiotic sensitivity testing to monitor MDR strains.
-
Reporting and surveillance of cases.
-
Mass vaccination campaigns in high-risk areas.
MCQs
-
Enteric fever is caused by:
A. E. coli
B. Vibrio cholerae
C. Salmonella Typhi
D. Shigella
Answer: C -
Typhoid fever is caused by:
A. Salmonella Typhi
B. Salmonella Paratyphi
C. Shigella
D. Campylobacter
Answer: A -
Salmonella Typhi is:
A. Gram-positive cocci
B. Gram-negative bacilli
C. Acid-fast bacilli
D. Spirochete
Answer: B -
The Vi antigen is a:
A. Flagellar antigen
B. Capsular antigen
C. Nuclear antigen
D. Ribosomal antigen
Answer: B -
Salmonella belongs to which family?
A. Vibrionaceae
B. Enterobacteriaceae
C. Neisseriaceae
D. Bacillaceae
Answer: B -
Main mode of transmission is:
A. Airborne
B. Fecal–oral
C. Vector-borne
D. Blood-borne
Answer: B -
A common source of infection is:
A. Mosquito bite
B. Contaminated water
C. Animal bite
D. Soil contact
Answer: B -
Chronic carriers usually harbor organisms in the:
A. Kidney
B. Gallbladder
C. Lung
D. Heart
Answer: B -
Enteric fever is common in areas with:
A. Good sanitation
B. Poor sanitation
C. Cold climate
D. High altitude
Answer: B -
Mechanical vector in transmission is:
A. Mosquito
B. Housefly
C. Tick
D. Louse
Answer: B -
Salmonella invades which intestinal structure?
A. Brunner’s glands
B. Peyer’s patches
C. Villi tips
D. Gastric glands
Answer: B -
Primary bacteremia occurs in the:
A. Early stage
B. Late stage
C. Recovery phase
D. Carrier stage
Answer: A -
Salmonella multiplies mainly inside:
A. RBCs
B. Macrophages
C. Platelets
D. Neurons
Answer: B -
Incubation period of enteric fever is:
A. 1–2 days
B. 3–5 days
C. 7–14 days
D. 30 days
Answer: C -
Classical fever pattern is:
A. Intermittent
B. Remittent
C. Step-ladder
D. Biphasic
Answer: C -
Rose spots appear during the:
A. 1st week
B. 2nd week
C. 3rd week
D. 4th week
Answer: B -
Relative bradycardia is called:
A. Murphy sign
B. Faget sign
C. Kernig sign
D. Romberg sign
Answer: B -
Most common symptom is:
A. Rash
B. Fever
C. Paralysis
D. Jaundice
Answer: B -
Intestinal perforation usually occurs in the:
A. 1st week
B. 2nd week
C. 3rd week
D. Recovery stage
Answer: C -
Most serious complication is:
A. Pneumonia
B. Intestinal perforation
C. Arthritis
D. Otitis
Answer: B -
Gold standard test in 1st week is:
A. Widal test
B. Stool culture
C. Blood culture
D. Urine test
Answer: C -
Most sensitive diagnostic method is:
A. Blood culture
B. Widal test
C. Bone marrow culture
D. ELISA
Answer: C -
Widal test detects antibodies against:
A. Capsule only
B. O and H antigens
C. Toxin
D. DNA
Answer: B -
Widal test becomes positive after:
A. 2 days
B. 4 days
C. 7–10 days
D. 1 month
Answer: C -
Stool culture is useful in:
A. 1st week
B. 2nd–3rd week
C. Before symptoms
D. Never
Answer: B -
Salmonella on MacConkey agar is:
A. Lactose fermenter
B. Non-lactose fermenter
C. Hemolytic
D. Swarming
Answer: B -
TSI reaction shows:
A. A/A
B. K/K
C. K/A with H2S
D. No reaction
Answer: C -
Leukocyte count is usually:
A. Increased
B. Decreased
C. Very high
D. Extremely high
Answer: B -
Drug commonly used is:
A. Penicillin
B. Ceftriaxone
C. Metronidazole
D. Acyclovir
Answer: B -
MDR typhoid means resistance to:
A. One drug
B. Two drugs
C. First-line drugs
D. All antibiotics
Answer: C -
Supportive therapy includes:
A. ORS
B. Insulin
C. Steroids in all cases
D. Antifungals
Answer: A -
Main preventive measure is:
A. Isolation only
B. Sanitation
C. Bed rest
D. Vitamins
Answer: B -
Vi polysaccharide vaccine is given after:
A. Birth
B. 6 months
C. 2 years
D. 10 years
Answer: C -
Oral live typhoid vaccine is:
A. BCG
B. Ty21a
C. OPV
D. MMR
Answer: B -
Typhoid conjugate vaccine can be given from:
A. Birth
B. 6 months
C. 5 years
D. 12 years
Answer: B -
Most common organ enlargement is:
A. Kidney
B. Spleen
C. Pancreas
D. Brain
Answer: B -
Chronic carrier detection is done by:
A. Blood test
B. Stool culture
C. Urine dipstick
D. X-ray
Answer: B -
Endotoxin of Salmonella is:
A. Protein toxin
B. Lipopolysaccharide
C. Enzyme
D. Capsule
Answer: B -
Paratyphoid fever is usually:
A. More severe
B. Milder
C. Always fatal
D. Chronic
Answer: B -
Rose spots are seen on:
A. Face
B. Trunk
C. Hands
D. Feet
Answer: B -
Major risk factor is:
A. Cold weather
B. Poor hygiene
C. Exercise
D. Sunlight
Answer: B -
Intestinal hemorrhage is due to:
A. Liver damage
B. Peyer’s patch ulceration
C. Kidney failure
D. Platelet excess
Answer: B -
Bone marrow culture remains positive even after:
A. Vaccination
B. Antibiotic therapy
C. Recovery
D. Carrier state ends
Answer: B -
Enteric fever primarily affects:
A. Respiratory system
B. Gastrointestinal system
C. Nervous system
D. Skin only
Answer: B -
Early constipation later changes to:
A. Vomiting
B. Diarrhea
C. Paralysis
D. Jaundice
Answer: B -
Step-ladder fever means:
A. Sudden rise and fall
B. Gradual daily rise
C. Biphasic fever
D. Intermittent fever
Answer: B -
Antibiotic sensitivity testing is done by:
A. Widal test
B. Culture
C. ELISA
D. PCR
Answer: B -
Gallbladder involvement is important in:
A. Acute phase
B. Carrier state
C. Recovery
D. Vaccination
Answer: B -
Most effective long-term prevention is:
A. Antibiotics alone
B. Vaccination only
C. Improved sanitation
D. Bed rest
Answer: C -
Enteric fever is also known as:
A. Cholera
B. Dysentery
C. Typhoid fever
D. Malaria
Answer: C