
Introduction
- Distilled water is the purest form of water commonly used in laboratories, hospitals, and industries.
- It is completely free from salts, minerals, organic impurities, and microorganisms.
- Every clinical and biochemistry laboratory requires distilled water for preparing reagents, cleaning glassware, and performing accurate diagnostic tests.
- The process of obtaining distilled water is known as distillation, which involves boiling water to produce steam and condensing it back into liquid form.
- This technique ensures only pure water molecules are collected, leaving behind all contaminants.
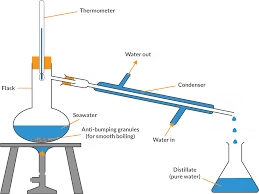
Principle of Distillation
- The distillation process is based on evaporation and condensation.
- When impure water is boiled, it turns into vapour, while the dissolved solids and impurities remain in the container.
- The steam is then cooled and converted back into liquid form — this condensed liquid is distilled water.
Thus, distillation separates volatile and non-volatile components based on their boiling points.

Instruments and Equipment
1. Distillation Unit (Water Still)
-
- The distillation unit is the main apparatus used for the preparation of distilled water.
- It includes all essential parts such as the boiler, condenser, receiver, and heating source combined in one setup.
Types of Distillation Units:
-
-
Single Distillation Unit: Produces water of standard purity suitable for general laboratory use.
-
Double Distillation Unit: Used when ultra-pure water is required for analytical or research purposes.
-
Construction: Usually made of borosilicate glass or stainless steel for durability and chemical resistance.
Function: To convert impure water into steam and condense it back to liquid distilled water.
2. Boiler or Boiling Chamber
The boiler (or boiling chamber) is the part of the unit where the actual evaporation takes place.
Features:
-
-
Holds the raw tap water before distillation.
-
Equipped with a heating coil or burner at the bottom.
-
Made from heat-resistant glass or stainless steel to withstand high temperatures.
-
Includes a safety valve to release excess steam pressure.
-
Function: Heats water to produce steam while leaving behind impurities such as salts, suspended particles, and organic contaminants.
3. Heating Element
-
- The heating element provides the heat energy needed for the distillation process.
- Modern units generally use electric heating coils, while older systems may use Bunsen burners or gas flames.
Function: Converts electrical energy into heat to boil water efficiently and consistently.
Note: Automatic thermostatic controls prevent overheating and maintain a constant boiling rate.
4. Condenser (Cooling Chamber)
The condenser plays a critical role in converting steam back into liquid water through cooling.
Construction:
-
-
Made up of a coiled glass or metal tube placed within a water jacket.
-
Cooling water continuously flows through the jacket, keeping the temperature low.
-
Cold water enters from the bottom inlet and exits from the top outlet, ensuring maximum cooling efficiency.
-
Function: As hot steam passes through the coiled tube, it loses heat to the cooling water and condenses into pure liquid water.
5. Receiver or Collection Flask
The receiver flask collects the condensed distilled water coming out from the condenser.
Features:
-
-
Made of borosilicate glass or HDPE (high-density polyethylene).
-
Must be clean, sterile, and airtight to prevent contamination.
-
The first 25–50 mL of distillate is usually discarded because it may contain volatile impurities.
-
Function: Safely collects and stores freshly distilled water immediately after condensation.
6. Thermometer or Thermostatic Control
A thermometer or automatic thermostat helps monitor and control the temperature of the distillation process.
Function:
-
-
Ensures water reaches boiling point (around 100°C).
-
Prevents overheating and possible damage to the apparatus.
-
Helps in maintaining consistent operation for uniform steam production.
-
7. Cooling Water Inlet and Outlet Tubes
These are rubber or PVC tubes that carry cold water into and out of the condenser.
Function:
-
-
Maintain a steady cooling effect during condensation.
-
The inlet tube supplies cold water from the bottom, and the outlet tube removes warm water from the top.
-
Some units include flow-control valves to regulate cooling speed and efficiency.
-
8. Storage Container
After collection, distilled water must be stored properly to maintain purity.
Requirements:
-
-
Use clean, airtight containers made of amber-colored glass or high-grade plastic (HDPE or polypropylene).
-
Store away from sunlight, dust, and chemical fumes.
-
Label each container clearly with “Distilled Water – Date of Preparation”.
-
Replace stored water weekly to prevent microbial growth.
-
Avoid metal containers, as distilled water can leach ions from the surface.
-
Function: Keeps distilled water free from contamination during storage and usage.
9. Deionizer Unit
A deionizer unit is sometimes connected either before or after the distillation process to achieve ultra-pure water.
Components:
-
-
Contains cation and anion exchange resins that remove charged ions (like Na⁺, Ca²⁺, Cl⁻, and SO₄²⁻).
-
Often used in research or clinical laboratories where high-precision tests are performed (e.g., spectrophotometry or chromatography).
-
Function: Ensures removal of all ionic impurities for the highest grade of purity.
10. Safety and Supporting Parts
To ensure smooth and safe operation, several accessories are attached to the distillation system.
Common Accessories:
-
-
Safety valve: Releases extra pressure from the boiling chamber.
-
Drain plug: Allows removal of leftover water and scale deposits.
-
Clamps and stands: Hold the glass apparatus firmly during heating.
-
Rubber stoppers and joints: Prevent vapor leakage.
-
Function: Prevent accidents, maintain tight connections, and ensure system stability.
| Instrument/Part | Function | Common Material |
|---|---|---|
| Distillation Unit | Complete setup for distillation | Glass / Stainless steel |
| Boiler | Boils impure water to form steam | Glass / Metal |
| Heating Element | Provides heat to boil water | Electric coil / Burner |
| Condenser | Cools and condenses steam into water | Glass / Copper |
| Cooling Tubes | Carry cooling water | Rubber / PVC |
| Receiver Flask | Collects distilled water | Glass / Plastic |
| Thermometer | Measures temperature | Mercury / Digital |
| Storage Bottle | Stores distilled water | Amber glass / HDPE |
| Deionizer | Removes ions for high purity | Resin column |
| Safety Parts | Maintain safe operation | Metal / Rubber |
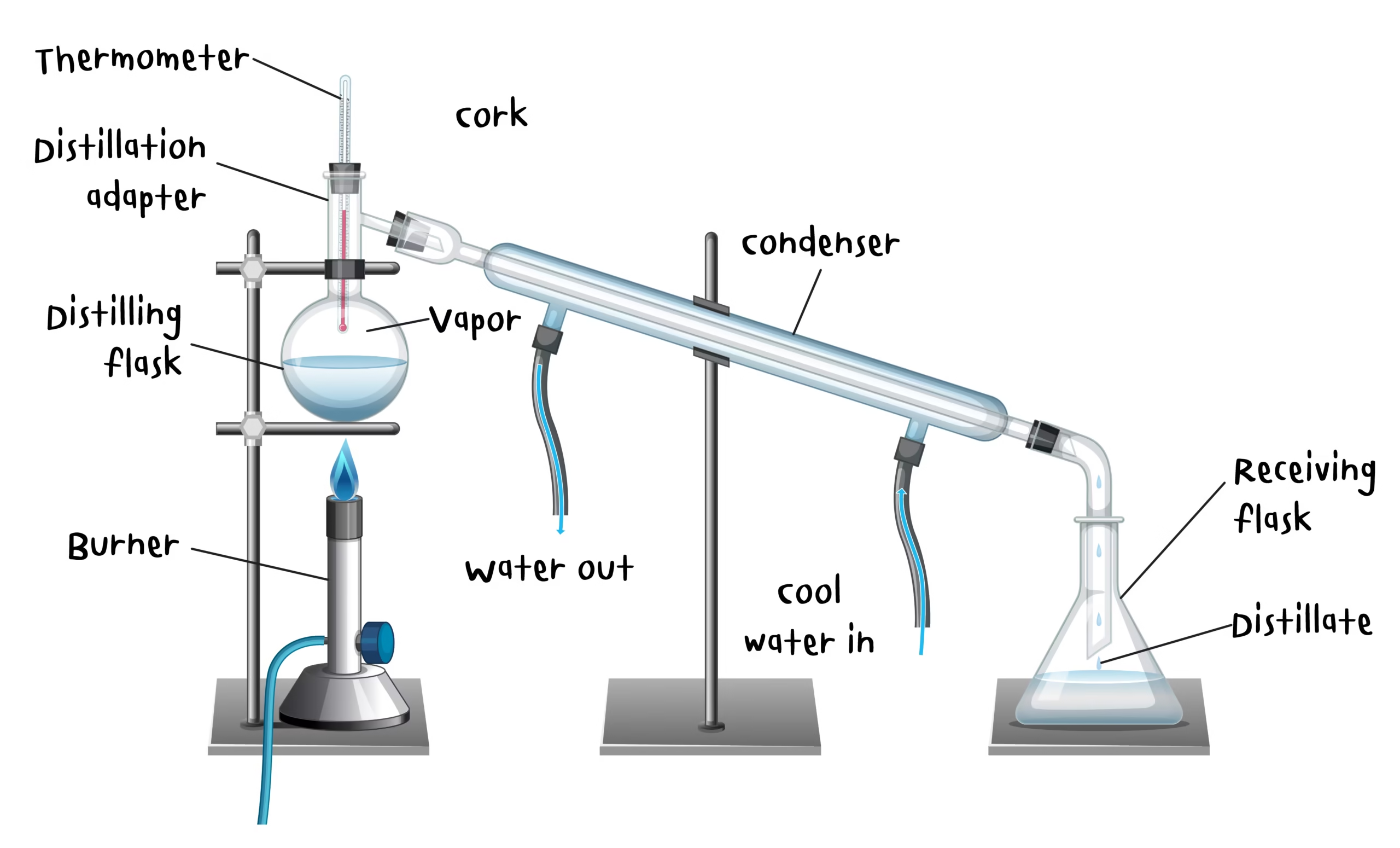
Procedure
-
Filling the Boiler: Pour clean tap water or filtered water into the distillation unit’s boiling chamber.
-
Heating: Start the heating element; water begins to boil and produces steam.
-
Steam Passage: Steam rises and passes through the condenser coil.
-
Condensation: Cooling water in the condenser helps convert steam back into liquid form.
-
Collection: The condensed water is collected in a clean receiver flask or bottle.
-
Discarding Initial Fraction: The first 25–50 mL of distillate should be discarded to remove volatile contaminants.
-
Storage: Transfer the pure water into clean, labelled storage bottles.
Storage of Distilled Water
Proper storage is essential to maintain the purity and prevent re-contamination.
-
Use clean, sterile glass or high-grade plastic containers (HDPE or borosilicate glass).
-
Keep containers tightly capped and away from direct sunlight.
-
Prefer amber-colored bottles to minimise light exposure.
-
Label containers with “Distilled Water – Date of Preparation.”
-
Replace stored water weekly to avoid microbial growth.
-
Avoid metal containers, as distilled water can corrode metal surfaces and leach ions.
Quality Control Tests
| Parameter | Ideal Range / Standard | Purpose |
|---|---|---|
| Conductivity | < 10 µS/cm | Indicates purity level; higher value = contamination |
| pH | 5.5 – 7.0 | Should be near neutral; slightly acidic due to CO₂ absorption |
| Appearance | Clear and colorless | No turbidity or suspended particles |
| Microbial Load | Nil | Ensures sterility for lab use |
Regular cleaning of the distillation unit with dilute acid (e.g., HCl) or laboratory detergent helps remove scale or salt deposits.
Applications
Distilled water is essential for multiple laboratory and industrial purposes:
-
Preparation of reagents, buffers, and control solutions
-
Cleaning glassware and instruments
-
Calibration of analyzers and pipettes
-
Autoclave and sterilizer operation
-
Battery water and coolant preparation
-
Pharmaceutical and cosmetic formulations
Precautions
-
Always use freshly prepared distilled water for analytical work.
-
Never reuse old chemical containers for storage.
-
Clean the distillation unit regularly to prevent contamination.
-
Ensure proper cooling water flow in the condenser during distillation.
-
Use distilled water only after confirming purity by conductivity and pH tests.