
Introduction
-
Flame photometry is an analytical technique used to determine the concentration of metal ions, primarily those of alkali and alkaline earth metals.
-
It is based on the principle that metal ions emit light of specific wavelengths when excited in a flame.
-
When the sample solution is introduced into the flame, the solvent evaporates, and the metal ions are converted into excited atoms.
-
These excited atoms emit light as they return to their ground state, and the intensity of this light is proportional to the element’s concentration.
-
It is commonly used to determine the concentrations of sodium, potassium, calcium, and lithium in biological, agricultural, and industrial samples.
-
The technique is simple, rapid, inexpensive, and widely applied in clinical biochemistry, soil testing, and quality control laboratories.
Working Principle
-
The working principle of flame photometry is based on the emission of light by atoms that are excited in a flame.
-
When a solution containing metallic salts is introduced into the flame, the solvent evaporates, leaving fine particles of the metallic salt.
-
The heat of the flame converts these metallic particles into free atoms and excites their outer electrons to higher energy levels.
-
When the excited electrons return to their ground state, they emit light of a characteristic wavelength specific to each element.
-
The intensity of the emitted light is directly proportional to the concentration of that element in the sample.
-
A detector measures this emitted light, and the signal is converted into a quantitative value using a calibration curve.
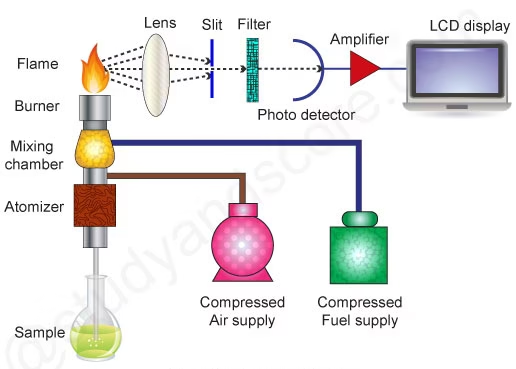
Parts of a Flame Photometer
-
Flame (Burner):
Provides the source of energy for excitation of atoms. A mixture of fuel (like propane or butane) and oxidant (air) produces a stable flame for atomization. -
Nebulizer:
Converts the liquid sample into a fine mist or aerosol, allowing it to be carried into the flame for analysis. -
Atomizer:
Helps in breaking down the sample into free atoms within the flame where excitation occurs. -
Optical System (Lens and Filters):
Focuses and selects the specific wavelength of light emitted by the excited atoms of the element being analyzed. Filters ensure only the desired wavelength reaches the detector. -
Detector (Photocell or Photomultiplier Tube):
Detects the intensity of emitted light and converts it into an electrical signal proportional to the concentration of the element. -
Readout Device (Galvanometer or Digital Display):
Displays the output in readable form—usually as a numerical value corresponding to the concentration of the element. -
Air-Fuel Control System:
Regulates the flow of gas and air to maintain a stable flame, which is essential for consistent readings.

Working Procedure
- Preparation of the Sample:
- The sample (usually in liquid form) is prepared by dissolving the substance in distilled water or an appropriate solvent. For biological samples, such as blood or urine, dilution might be required to bring the ion concentrations within the instrument’s detection range.
- Nebulization:
- The liquid sample is aspirated into the nebulizer, which converts it into a fine aerosol mist.
- Atomization in the Flame:
- The aerosol is then introduced into the flame, usually fuelled by air and a combustible gas like propane or acetylene. The flame provides enough energy to excite the metal ions in the sample.
- Excitation of Ions:
- In the flame, the metal ions absorb energy and their electrons are excited to higher energy levels.
- Emission of Light:
- As the electrons return to their ground state, the ions emit light of specific wavelengths characteristic of the metal ion in the sample. For example, sodium ions emit light at 589 nm (yellow), while potassium ions emit light at 766 nm (red-violet).
- Detection of Light:
- The emitted light is passed through a monochromator, which isolates the light of a specific wavelength corresponding to the metal being analyzed. A photodetector detects the intensity of the emitted light.
- Measurement:
- The photodetector converts the light intensity into an electrical signal. The signal is amplified, and the ion concentration is displayed on a readout device. A calibration curve is usually created using standard solutions of known concentrations for accurate quantification.
- Result Interpretation:
- The concentration of the metal ion in the sample is directly proportional to the intensity of the light emitted and can be read from the instrument.
Advantages
- Simplicity and Ease of Use:
- The operation of flame photometers is straightforward, making it easy for technicians to run routine analyses with minimal training.
- Quick and Accurate:
- Provides rapid results with high accuracy for detecting and quantifying alkali and alkaline earth metals, such as sodium, potassium, calcium, and lithium.
- Cost-Effective:
- Flame photometry is an economical analytical technique with relatively low equipment and operating costs compared to more complex techniques like atomic absorption spectroscopy.
- Minimal Sample Preparation:
- Requires minimal sample preparation compared to other methods, reducing the time needed for analysis.
- Wide Range of Applications:
- Useful in clinical labs, food industries, environmental testing, and pharmaceutical industries to analyse metal ions.
Disadvantages
- Limited to Certain Elements:
- Only works for alkali metals (e.g., sodium, potassium) and alkaline earth metals (e.g., calcium). It is not suitable for analyzing transition metals or non-metals.
- Lower Sensitivity for Some Elements:
- Flame photometry may not detect very low concentrations of metal ions, and more sensitive techniques (e.g., atomic absorption spectroscopy) may be needed for trace analysis.
- Interference:
- Other ions or contaminants in the sample can interfere with the detection, leading to inaccuracies. For instance, other metals can cause spectral overlap or chemical interference in the flame.
- Requires Calibration:
- The accuracy of flame photometry is highly dependent on the preparation of standard solutions and frequent calibration to ensure reliability.
- Flame Instability:
- Variations in flame temperature or composition can lead to inconsistent results, requiring close monitoring during operation.
- Non-Specific Detection:
- The method does not provide information about the chemical form of the metal ion or distinguish between different oxidation states.
- Ion concentration.
Uses
- Electrolyte Analysis:
- Flame photometry is commonly used to measure sodium (Na⁺) and potassium (K⁺) levels in biological fluids like blood and urine, essential for diagnosing conditions like electrolyte imbalance, kidney function, and heart disorders.
- Clinical Diagnosis:
- The technique is widely used in clinical biochemistry labs for detecting disorders related to electrolyte disturbances, such as hyponatremia (low sodium levels) and hyperkalemia (high potassium levels).
- Calcium and Magnesium Estimation:
- It can also be used to quantitatively analyze calcium and magnesium in biological fluids, which are crucial for bone health and muscle function.
- Soil and Water Testing:
- Although primarily used in biochemistry, flame photometry is also applied in environmental science for testing the concentration of minerals like sodium and potassium in soil and water.
- Pharmaceutical and Food Industry:
- It is used to analyze minerals in pharmaceutical formulations and to ensure quality control in food products by measuring their metal ion content.
MCQs
-
Flame photometry is based on which principle?
A. Absorption of light
B. Emission of light
C. Reflection of light
D. Scattering of light -
Flame photometry is also known as:
A. Flame emission spectrophotometry
B. Atomic absorption spectrophotometry
C. Fluorimetry
D. Nephelometry -
Which elements are commonly estimated by flame photometry?
A. Sodium and potassium
B. Iron and copper
C. Zinc and lead
D. Silver and mercury -
In flame photometry, the intensity of emitted light is:
A. Inversely proportional to concentration
B. Directly proportional to concentration
C. Independent of concentration
D. Depends on wavelength only -
The sample is introduced into the flame as:
A. Powder
B. Vapor
C. Fine mist (aerosol)
D. Solid -
Which part of the instrument converts liquid sample into fine droplets?
A. Atomizer
B. Nebulizer
C. Burner
D. Filter -
What is the function of the burner in flame photometry?
A. Detects light
B. Provides excitation energy
C. Converts light to electricity
D. Measures concentration -
The optical filter in a flame photometer:
A. Produces light
B. Selects specific wavelength
C. Converts light to current
D. Mixes gases -
The detector used in a flame photometer is usually a:
A. Photomultiplier tube
B. Thermocouple
C. Cathode ray tube
D. Photographic plate -
Which of the following gas mixtures is commonly used in the flame?
A. Acetylene–oxygen
B. Hydrogen–oxygen
C. Propane–air
D. Nitrogen–air -
The main limitation of flame photometry is:
A. Can detect only non-metals
B. Can detect only metals that emit in visible range
C. Requires solid samples
D. Cannot measure concentration -
The wavelength of emitted light is characteristic of:
A. Type of solvent
B. Element present
C. Concentration of sample
D. Temperature of flame -
Which of the following elements gives yellow color in flame?
A. Sodium
B. Potassium
C. Calcium
D. Lithium -
Potassium imparts what color to the flame?
A. Blue
B. Red
C. Violet
D. Green -
Calcium gives what color in the flame test?
A. Brick red
B. Apple green
C. Blue
D. Yellow -
Lithium gives which flame color?
A. Crimson red
B. Yellow
C. Green
D. Orange -
The calibration curve in flame photometry is plotted between:
A. Absorbance vs concentration
B. Emission intensity vs concentration
C. Voltage vs wavelength
D. Temperature vs time -
Which of the following is NOT a part of a flame photometer?
A. Nebulizer
B. Burner
C. Monochromator
D. Detector -
The solvent used in flame photometry should be:
A. Highly viscous
B. Volatile and pure
C. Non-volatile
D. Colored solution -
In clinical biochemistry, flame photometry is used to estimate:
A. Glucose
B. Sodium and potassium
C. Urea
D. Creatinine -
The purpose of diluting samples is to:
A. Reduce viscosity and avoid clogging
B. Increase sensitivity
C. Improve flame temperature
D. Change emission wavelength -
The main source of error in flame photometry is due to:
A. Incorrect wavelength selection
B. Unstable flame and contamination
C. Too high sample concentration
D. Overheating of detector -
The unit of emission intensity is generally expressed as:
A. nm
B. V (volts)
C. Arbitrary units (a.u.)
D. Moles per liter -
The flame photometer cannot be used for:
A. Sodium
B. Potassium
C. Magnesium
D. Calcium -
The excitation source in flame photometry is:
A. UV lamp
B. Electric current
C. Flame
D. Laser -
The function of air-fuel control system is to:
A. Control wavelength
B. Maintain stable flame
C. Measure temperature
D. Detect emission -
Which type of analysis is performed by flame photometry?
A. Qualitative only
B. Quantitative only
C. Both qualitative and quantitative
D. Neither -
The color produced in flame is due to:
A. Ionization
B. Excitation of electrons
C. Combustion of gases
D. Vibration of molecules -
The flame temperature affects:
A. Sensitivity of measurement
B. Color of the flame only
C. Wavelength of emission only
D. Calibration curve shape -
To ensure accuracy, the instrument should be calibrated using:
A. Unknown samples
B. Distilled water
C. Standard solutions
D. Buffer solutions
Answer
-
B
-
A
-
A
-
B
-
C
-
B
-
B
-
B
-
A
-
C
-
B
-
B
-
A
-
C
-
A
-
A
-
B
-
C
-
B
-
B
-
A
-
B
-
C
-
C
-
C
-
B
-
C
-
B
-
A
-
C