
Introduction
-
Atomic Absorption Spectroscopy (AAS) is an analytical technique used to determine the concentration of specific metal elements in a sample by measuring the absorption of light by free atoms.
-
It is based on the principle that ground-state atoms absorb light of specific wavelengths, and the amount of absorbed light is directly proportional to the concentration of the element.
-
In AAS, the sample is atomized—usually in a flame or graphite furnace—so that the elements are present in their atomic form.
-
A light beam from a hollow cathode lamp, specific for the element being analyzed, passes through the vaporized sample, and the absorption is measured.
-
Atomic Absorption Spectroscopy is widely used in clinical biochemistry, environmental monitoring, food analysis, and industrial quality control to detect trace metals like copper, zinc, lead, and iron.
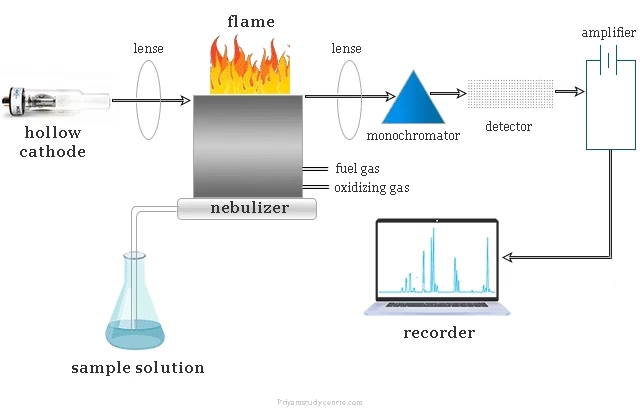
Working principle
-
The working principle of Atomic Absorption Spectroscopy (AAS) is based on the absorption of light by free atoms in the ground state.
-
When a sample containing metallic ions is atomized in a flame or graphite furnace, the ions are converted into free atoms.
-
A hollow cathode lamp emits light of a wavelength characteristic of the element being analyzed.
-
These free atoms in the sample absorb part of this light, and the amount of light absorbed is directly proportional to the concentration of the element present.
-
The remaining light passes to a detector, which measures the decrease in intensity (absorbance), allowing calculation of the element’s concentration using Beer–Lambert’s law.
Parts of Atomic Absorption Spectroscopy
Radiation Source (Hollow Cathode Lamp)
-
The hollow cathode lamp (HCL) is the most common light source used in AAS.
-
It consists of a cathode made from the metal of the element to be analyzed and an anode sealed in a glass tube filled with an inert gas (usually neon or argon).
-
When a high voltage is applied, the inert gas gets ionized, and these ions strike the cathode, causing atoms of the metal to be ejected.
-
These atoms emit radiation of specific wavelengths characteristic of that metal.
-
Each element requires a specific lamp (e.g., sodium lamp for Na, copper lamp for Cu, etc.), ensuring accurate wavelength selection.
Atomizer (Flame or Graphite Furnace)
-
The atomizer converts the sample into free atoms (neutral atoms) suitable for light absorption.
-
Two main types are used:
-
Flame Atomizer:
-
Uses a flame (air-acetylene or nitrous oxide-acetylene) to evaporate solvent and dissociate molecules into free atoms.
-
It is simple, economical, and suitable for moderate concentration ranges.
-
-
Graphite Furnace Atomizer (Electrothermal AAS):
-
The sample is placed inside a graphite tube and electrically heated in steps — drying, ashing, and atomization.
-
Provides much higher sensitivity and requires only microliter samples.
-
-
Nebulizer and Burner System
-
The nebulizer converts the liquid sample into a fine mist or aerosol.
-
This aerosol is carried by a gas (usually air or acetylene) into the burner, where atomization takes place.
-
The burner head provides a long, narrow flame (usually 10 cm), allowing the light beam to pass through the region with the highest concentration of free atoms.
-
Proper alignment of the burner and flame height is crucial for maximum absorbance and reproducibility.
Monochromator
-
The monochromator isolates the specific wavelength of light absorbed by the element and removes unwanted radiation from other wavelengths.
-
It typically consists of diffraction gratings or prisms that separate light based on wavelength.
-
This ensures that only the resonance line of the element being studied reaches the detector, enhancing accuracy and selectivity.
Detector (Photomultiplier Tube – PMT)
-
The detector measures the intensity of light that passes through the atomized sample.
-
A photomultiplier tube is most commonly used because it is highly sensitive to low light levels.
-
It converts the light signal into an electrical current.
-
The difference in light intensity before and after the sample represents the amount of light absorbed, which is directly proportional to the concentration of the analyte.
Signal Processor and Readout Device
-
The weak electrical signal from the detector is amplified by an electronic amplifier.
-
The resulting signal is processed by the instrument’s computer or analog circuits to calculate absorbance or concentration.
-
The results are displayed on a digital screen, recorded on paper, or stored electronically.
-
Modern AAS instruments also provide calibration curves and data logging features.
Optical System (Lenses and Mirrors)
-
-
The optical system aligns and focuses the radiation path between the light source, atomizer, monochromator, and detector.
-
Mirrors and lenses ensure that the maximum amount of light passes through the atomized zone for accurate absorption measurement.
-
Proper optical alignment is essential to minimize stray light and maintain high precision.
-
Working Procedure
- Preparation of the Sample:
- The sample (e.g., a solution) is prepared, often by dissolving it in an appropriate solvent to ensure that the metal ions are in a measurable form.
- Nebulization:
- The liquid sample is introduced into the nebulizer, which converts the sample into an aerosol.
- Atomization:
- The aerosol or liquid sample is introduced into the atomizer (either a flame or a graphite furnace). The heat breaks down the sample into its constituent atoms.
- Light Absorption:
- A hollow cathode lamp specific to the tested metal emits light at a particular wavelength. As this light passes through the cloud of atoms in the atomizer, some of the light is absorbed by the free atoms, causing electrons to jump to higher energy levels.
- Detection:
- The monochromator isolates the wavelength of light absorbed, and the detector measures the intensity of the remaining light. The decrease in intensity correlates with the concentration of the element in the sample.
- Data Interpretation:
- The electrical signal from the detector is converted into a concentration value, which is displayed on the readout device.
Uses
- Trace Metal Analysis:
- AAS is used to detect trace amounts of metals like lead (Pb), copper (Cu), zinc (Zn), calcium (Ca), and magnesium (Mg) in biological samples such as blood, urine, and tissues.
- Clinical Diagnostics:
- Measures essential elements (e.g., iron, magnesium) or toxic elements (e.g., lead, mercury) in human samples to diagnose deficiencies or toxicities.
- Pharmaceuticals:
- Ensures quality control by determining trace metal impurities in pharmaceutical products.
- Environmental Testing:
- Used for detecting heavy metals in water, soil, and air to assess pollution levels.
- Food Industry:
- Determines the metal content in food products to ensure safety and compliance with regulatory standards.
Advantages
- High Sensitivity:
- AAS can detect trace levels of metals, making it suitable for analyzing very low concentrations (down to parts per billion).
- Element-Specific:
- Each hollow cathode lamp is specific to one element, ensuring high specificity for the target metal.
- Wide Range of Elements:
- AAS can be used to measure a wide variety of metal elements, from essential nutrients to toxic heavy metals.
- Precision and Accuracy:
- Provides accurate and reproducible measurements of metal ion concentrations.
- Simple Sample Preparation:
- Requires minimal sample preparation, especially when analyzing liquid samples.
Disadvantages
- Single-Element Analysis:
- AAS can only measure one element at a time. The sample must be run multiple times for multi-element analysis, each with a different hollow cathode lamp.
- Limited to Metals:
- AAS is mainly used for metals and cannot measure non-metals or organic compounds.
- Sample Destruction:
- In flame AAS, the sample is destroyed during analysis, making it unsuitable for precious or limited samples.
- Interference:
- Chemical and spectral interferences can affect the accuracy of the measurement. For example, other substances in the sample might absorb light at similar wavelengths, leading to erroneous results.
- Cost:
- AAS instruments, particularly graphite furnace systems, can be expensive to purchase and maintain.
- Low Throughput:
- Since AAS is a single-element technique, it can be time-consuming if multiple elements need to be analysed in the same sample.
MCQs
-
Atomic Absorption Spectroscopy (AAS) is used to determine:
A. Non-metal concentration
B. Metal ion concentration
C. Gas composition
D. Organic compounds -
The basic principle of AAS is:
A. Absorption of light by free atoms
B. Emission of light by atoms
C. Scattering of light by particles
D. Reflection of light -
The light source used in AAS is:
A. Tungsten lamp
B. Hollow cathode lamp
C. Mercury vapor lamp
D. Xenon lamp -
AAS measures the amount of:
A. Light emitted by excited atoms
B. Light absorbed by ground-state atoms
C. Fluorescence from atoms
D. Electrons released from atoms -
The atomizer in AAS converts:
A. Atoms into molecules
B. Ions into free atoms
C. Molecules into ions
D. Solids into gases directly -
The flame in AAS serves to:
A. Dissolve the sample
B. Atomize the sample
C. Measure wavelength
D. Cool the system -
The most commonly used flame in AAS is:
A. Acetylene-air flame
B. Hydrogen-oxygen flame
C. Butane-air flame
D. Methane-oxygen flame -
The graphite furnace in AAS is used for:
A. Increasing light intensity
B. Measuring high concentrations
C. Trace metal analysis
D. Cooling the flame -
Which gas combination produces the hottest flame in AAS?
A. Acetylene-air
B. Acetylene-nitrous oxide
C. Hydrogen-air
D. Methane-oxygen -
The process of converting liquid samples into mist is called:
A. Ionization
B. Nebulization
C. Evaporation
D. Condensation -
The function of the monochromator in AAS is to:
A. Produce light
B. Separate different wavelengths
C. Focus the light beam
D. Measure absorbance directly -
Which detector is commonly used in AAS?
A. Photodiode
B. Thermocouple
C. Photomultiplier tube
D. Cathode ray tube -
Beer–Lambert’s law states that absorbance is:
A. Inversely proportional to concentration
B. Directly proportional to concentration
C. Independent of path length
D. Not related to concentration -
The absorption wavelength is characteristic of:
A. Solvent used
B. Atom of the element
C. Flame temperature
D. Detector type -
Each element in AAS requires:
A. The same hollow cathode lamp
B. A separate hollow cathode lamp
C. A tungsten filament
D. A mercury lamp -
The role of the nebulizer in AAS is to:
A. Produce plasma
B. Convert sample to aerosol
C. Separate elements
D. Measure light intensity -
Which of the following elements is commonly analyzed by AAS?
A. Sodium (Na)
B. Calcium (Ca)
C. Copper (Cu)
D. All of the above -
The unit of absorbance used in AAS is:
A. Moles per liter
B. Arbitrary unit (A.U.)
C. Joules
D. Lux -
The background correction in AAS is done using:
A. Deuterium lamp
B. Sodium lamp
C. Argon lamp
D. Xenon lamp -
AAS is primarily a:
A. Qualitative technique
B. Quantitative technique
C. Separation technique
D. Chromatographic technique -
The atomization process in AAS involves:
A. Evaporation and dissociation
B. Ionization and recombination
C. Polymerization
D. Condensation -
Which of the following can cause errors in AAS?
A. Unstable flame
B. Incorrect wavelength
C. Contaminated samples
D. All of the above -
The optical system in AAS contains:
A. Lenses and mirrors
B. Burners only
C. Filters only
D. None -
The absorbance is measured at:
A. Ultraviolet or visible region
B. Infrared region
C. Microwave region
D. X-ray region -
The calibration curve in AAS is plotted as:
A. Absorbance vs Wavelength
B. Absorbance vs Concentration
C. Intensity vs Time
D. Concentration vs Temperature -
The main difference between flame AAS and graphite furnace AAS is:
A. Type of light source
B. Type of atomizer
C. Type of detector
D. Type of sample used -
The hollow cathode lamp operates under:
A. High vacuum
B. Inert gas atmosphere
C. Hydrogen gas
D. Oxygen gas -
In AAS, the element absorbs light of:
A. Any wavelength
B. Its own characteristic wavelength
C. The shortest wavelength
D. Longest wavelength -
Which law forms the basis of AAS measurements?
A. Avogadro’s law
B. Beer–Lambert’s law
C. Boyle’s law
D. Dalton’s law -
AAS is most suitable for determining:
A. Non-metal elements
B. Trace metals in liquid samples
C. Organic molecules
D. Gaseous elements
✅ Answer Key
-
B
-
A
-
B
-
B
-
B
-
B
-
A
-
C
-
B
-
B
-
B
-
C
-
B
-
B
-
B
-
B
-
D
-
B
-
A
-
B
-
A
-
D
-
A
-
A
-
B
-
B
-
B
-
B
-
B
-
B
