
Introduction
Sterilization describes a process that destroys or eliminates all forms of microbial life and is carried out in health-care facilities by physical or chemical methods.
Disinfection: Disinfection describes a process that eliminates many or all pathogenic microorganisms, except bacterial spores, on inanimate objects.
Cleaning: Cleaning removes visible soil (e.g., organic and inorganic material) from objects and surfaces. It is normally accomplished manually or mechanically using water with detergents or enzymatic products.
Decontamination: Decontamination removes pathogenic microorganisms from objects so they are safe to handle, use, or discard.
The various methods of sterilization are: 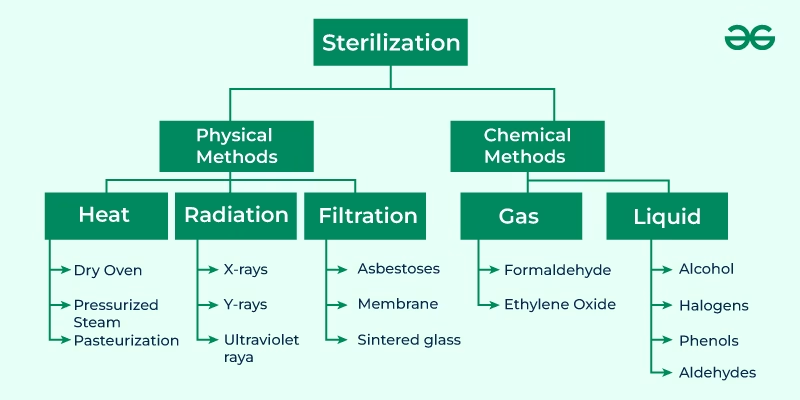
- Physical Method
- Heat methods
- Dry heat
- Incineration
- Red heat
- Flaming
- Hot air oven
- Moist heat
- Dry saturated steam – Autoclaving
- Boiling water/ steam at atmospheric pressure
- Hot water below boiling point
- Dry heat
- Radiation method
- Ionizing
- Gamma radiation
- X-ray radiation
- Non-ionizing
- UV radiation
- Ionizing
- Filtration method
- Membrane filtration
- HEPA filtration
- depth filtration
- Chemical Method
-
- Gaseous
- Ethylene oxide
- Hydrogen peroxide vapour
- formaldehyde
- Liquid
- Ethylene oxide
- Hydrogen peroxide
- Iodine
- Gaseous
Heat Sterilization
- Heat sterilization is the most widely used and reliable method, destroying enzymes and other essential cell constituents.
- The process is more effective in a hydrated state where hydrolysis and denaturation occur under high humidity conditions. Thus, lower heat input is required. Under a dry state, oxidative changes occur, requiring higher heat input.
- This method of sterilization can be applied only to the thermostable products. Still, it can be used for moisture-sensitive materials for dry heat (160 180°C) sterilization and for moisture-resistant materials for which moist heat (121-134°C) sterilization is used.
- The efficiency with which heat can inactivate microorganisms depends upon the degree of heat, the exposure time and the presence of water.
- The action of heat will be due to the induction of lethal chemical events mediated through the action of water and oxygen.
- In the presence of water, much lower temperature time exposures are required to kill microbes than in the absence of water.
- In this process, dry and moist heat are sterilised.
Dry Heat Sterilization:
Examples of Dry heat sterilization are:
- Incineration
- Red heat
- Flaming
- Hot air oven
Hot-air oven
Hot air ovens are versatile laboratory instruments designed primarily for drying, sterilization, and heating. Here’s a more detailed description of their features, components, and applications:
Features
- Temperature Range:
- Typically ranges from 50°C to 300°C, allowing for a variety of applications.
- Uniform Heat Distribution:
- Designed to ensure consistent temperature throughout the chamber, often using forced air circulation.
- Digital Control Panel:
- Equipped with an LCD or LED display for easy temperature and time settings, often featuring programmable options.
- Safety Mechanisms:
- Includes over-temperature protection, alarms, and sometimes a safety lock to prevent accidental opening.
- Insulated Construction:
- Built with heat-resistant materials to minimize heat loss and maintain stable internal temperatures.
Components
- Chamber:
- The main compartment where items are placed is usually made of stainless steel for durability and easy cleaning.
- Heating Elements:
- Electric heating elements located around the chamber provide the necessary heat for sterilization or drying.
- Fan System:
- In forced convection models, a fan circulates hot air for even heat distribution, reducing temperature gradients.
- Shelves/Racks:
- Adjustable shelves allow for flexibility in loading different sizes and quantities of materials.
- Control System:
- Digital or analog controls for setting and monitoring temperature and time, often with timers and alarms.
Applications
- Laboratory Sterilization: Commonly used in microbiology labs for sterilizing glassware and media.
- Pharmaceuticals: For drying powders and sterilizing containers.
- Food Industry: Used for drying food samples and sterilizing equipment.
- Material Testing: Heating materials for testing their properties under controlled conditions.
Moist Heat Sterilization:
Moist heat may be used in three forms to achieve microbial inactivation
- Dry saturated steam – Autoclaving
- Boiling water/ steam at atmospheric pressure
- Hot water below boiling point
Autoclave
An autoclave is a crucial piece of laboratory equipment used for sterilization. It utilizes high-pressure steam to eliminate microorganisms, spores, and viruses from various materials. Here’s a detailed overview:
Features
- High Pressure and Temperature:
- Operates at 121°C (250°F) at 15 psi for standard cycles, although settings can vary based on specific needs.
- Steam Generation:
- Uses steam to create a moist environment, which is more effective for killing organisms than dry heat.
- Control Systems:
- Equipped with digital displays for monitoring temperature, pressure, and cycle time. Some models offer programmable settings.
- Safety Features:
- Includes pressure release valves, door locks during operation, and alarms for over-pressure or temperature anomalies.
- Chamber Design:
- Made from durable materials like stainless steel to withstand high temperatures and pressures.
Components
- Sterilization Chamber:
- The main compartment where items are placed, designed to withstand high pressure.
- Heating Element:
- Heats the water to generate steam, ensuring the chamber reaches the required temperature.
- Pressure Control System:
- Manages and monitors the pressure within the chamber for safe operation.
- Door Mechanism:
- Often features a locking mechanism that prevents opening until the pressure is safely released.
- Drainage System:
- Allows for the removal of condensate and ensures that the chamber is ready for the next cycle.
Operative Procedure
1. Preparation
- Inspect the Autoclave: Check for any visible damage or leaks. Ensure the door seals are intact.
- Gather Materials: Collect items to be sterilized (e.g., instruments, media). Ensure they are suitable for autoclaving.
2. Loading
- Arrange Items: Place items in the autoclave chamber:
- Avoid overcrowding to allow steam circulation.
- Position containers with the opening facing down to prevent water accumulation.
- Use Appropriate Packaging: Wrap items in autoclave-safe materials (e.g., muslin, sterilization pouches) to allow steam penetration.
3. Setting the Parameters
- Close the Door: Ensure the door is securely locked.
- Select Cycle Type: Choose the appropriate sterilization cycle based on the sterilised materials (e.g., standard, liquid, or specific cycles for sensitive items).
- Set Temperature and Time: Typically, for most materials, set to 121°C (250°F) for at least 15-30 minutes, depending on the load.
4. Running the Cycle
- Start the Cycle: Press the start button and monitor the display for progress.
- Observe Pressure and Temperature: Ensure the autoclave reaches the desired parameters. Alarms should alert you to any issues.
5. Completion
- End of Cycle: Wait for the cycle to complete. The autoclave will usually indicate when it’s finished.
- Cooling Down: Allow the chamber to cool and depressurize. Do not open the door until the pressure gauge indicates it’s safe.
6. Unloading
- Open the Door Carefully: Slowly open the door to avoid steam burns once safe.
- Use Protective Gear: Wear gloves and safety glasses when handling sterilized items, as they may still be hot.
- Check Items: Inspect for proper sterilization (e.g., indicators changing colour in sterilization pouches).
7. Post-Operation
- Clean the Chamber: Wipe down the interior to remove any condensation or debris.
- Log the Cycle: Record details of the cycle in the lab logbook, including date, time, materials sterilized, and operator name.
- Schedule Maintenance: Regularly schedule maintenance checks based on usage.
Applications
- Medical and Dental Facilities: Sterilizing surgical instruments and dental tools.
- Microbiology Laboratories: Preparing media and sterilizing equipment to prevent contamination.
- Pharmaceuticals: Sterilizing products and materials for drug production.
- Research Institutions: Decontaminating equipment and biohazardous waste.
Radiation Methods
Radiation sterilization can be classified into two main categories: ionizing and non-ionizing radiation. Here’s an overview of both methods:
Ionizing Radiation Sterilization
Definition: Ionizing radiation has enough energy to remove tightly bound electrons from atoms, creating ions. This process effectively kills or inactivates microorganisms.
Types of Ionizing Radiation:
- Gamma Radiation:
- Source: Cobalt-60 or Cesium-137 isotopes.
- Applications: Sterilizes medical devices, pharmaceuticals, and food products.
- Mechanism: Penetrates deeply into materials, disrupting DNA and cellular structures.
- Electron Beam (E-beam) Radiation:
- Source: Electron accelerators.
- Applications: Surface sterilization of medical devices, packaging, and some food items.
- Mechanism: Produces high-energy electrons that kill microorganisms by damaging their DNA.
- X-ray Radiation:
- Source: X-ray machines.
- Applications: Sterilizing pharmaceuticals and sensitive medical devices.
- Mechanism: Similar to gamma rays, but with different energy levels and penetration capabilities.
Procedure for Ionizing Radiation Sterilization:
- Preparation: Assess materials and select appropriate radiation source.
- Packaging: Use radiation-permeable materials.
- Loading: Arrange items in the radiation chamber.
- Sterilization Cycle: Set parameters (dose and duration) and start the cycle.
- Monitoring: Use dosimeters to ensure proper radiation exposure.
- Completion and Unloading: Follow safety protocols when handling sterilized items.
Non-Ionizing Radiation Sterilization
Definition: Non-ionizing radiation does not have enough energy to remove electrons and generally does not cause ionization in biological tissues. It is less effective for sterilization compared to ionizing radiation.
Types of Non-Ionizing Radiation:
- Ultraviolet (UV) Radiation:
- Source: UV lamps emitting UV-C light.
- Applications: Surface sterilization of air, water, and certain equipment.
- Mechanism: Damages the DNA of microorganisms, inhibiting their ability to replicate.
- Microwave Radiation:
- Source: Microwave generators.
- Applications: Primarily used for heating and cooking but can assist in sterilization processes by raising temperatures.
- Mechanism: Causes water molecules to vibrate, generating heat that can kill microorganisms.
Procedure for Non-Ionizing Radiation Sterilization:
- Preparation: Identify items suitable for UV sterilization.
- Placement: Position items in the path of the UV light source.
- Sterilization Duration: Expose items to UV light for a specified time (usually 10-30 minutes, depending on intensity and distance).
- Monitoring: Ensure proper exposure time and intensity to achieve effective sterilization.
- Completion and Safety: Follow safety protocols to avoid UV exposure to skin and eyes.
Filtration Method
Filtration sterilization is a physical method to remove microorganisms, including bacteria and spores, from liquids and gases. It is particularly useful for heat-sensitive materials that cannot withstand traditional sterilization methods like autoclaving or radiation.
Types of Filtration
- Membrane Filtration:
- Description: Uses a porous membrane with a defined pore size (typically 0.2 micrometres or smaller) to remove particles and microorganisms physically.
- Applications: Sterilizing heat-sensitive liquids (e.g., culture media, pharmaceuticals) and air in controlled environments.
- Depth Filtration:
- Description: Utilizes a thick layer of fibrous or granular material to trap particles as the liquid passes through.
- Applications: Often used for pre-filtration of larger volumes or viscous fluids before membrane filtration.
- HEPA Filtration:
- Description: High-efficiency particulate Air (HEPA) filters can remove at least 99.97% of particles that are 0.3 micrometers in diameter.
- Applications: Used in cleanrooms, biological safety cabinets, and air purification systems to maintain sterile environments.
Procedure for Filtration Sterilization
1. Preparation
- Select Filtration Method: Choose between membrane, depth, or HEPA filtration based on sterilising material.
- Gather Equipment: Ensure you have the appropriate filter units, syringes, or filtration apparatus.
2. Setup
- Assemble Filtration System: Set up the filtration apparatus, ensuring it is clean and contaminant-free.
- Choose Filter: Select the appropriate filter with the required pore size for the specific application.
3. Filtration Process
- Sterilize Equipment: If applicable, autoclave or disinfect the filtration apparatus before use.
- Filter the Liquid or Gas:
- For liquids: Use a syringe or vacuum to push the liquid through the membrane filter.
- For gases: Ensure the air passes through the HEPA filter under the correct conditions.
4. Collection
- Collect Sterilized Output: Gather the filtered liquid or air in sterile containers or directly into the intended application.
- Check Integrity: Inspect the filters and system for any signs of leakage or failure.
5. Post-Operation
- Dispose of Used Filters: Follow appropriate biohazard waste disposal protocols for used filters.
- Document the Process: Record details such as date, type of filtration used, and batch number for traceability.
Chemical Agents for Sterilization
Chemical agents used for sterilization are essential in healthcare, laboratories, and various industries where maintaining sterility is critical. Here’s a list of common chemical agents and their characteristics:
-
Ethylene Oxide (EtO)
- Form: Gas at room temperature.
- Mechanism:
- Ethylene oxide penetrates packaging materials and the items being sterilized.
- It reacts with cellular components, particularly proteins and nucleic acids, through alkylation, disrupting their function and leading to cell death.
- Applications:
- Widely used to sterilize heat-sensitive medical instruments (e.g., endoscopes, surgical tools).
- Employed in the sterilization of some pharmaceuticals and implantable devices.
- Advantages:
- Highly effective against a broad spectrum of microorganisms, including bacterial spores.
- Can penetrate complex geometries and packaging materials.
- Considerations:
- Requires careful handling due to its toxicity and flammability.
- Items must be aerated post-sterilization to remove residual gas.
- Regulatory guidelines must be followed, and monitoring of exposure levels is essential.
-
Hydrogen Peroxide
- Form: Available as a liquid or vapour.
- Mechanism:
- Hydrogen peroxide generates free radicals that cause oxidative damage to proteins, lipids, and DNA, leading to microbial death.
- Applications:
- Used to sterilize surgical instruments and dental tools and as a surface disinfectant.
- In vapour form, it can sterilize large areas or enclosed spaces (e.g., operating rooms).
- Advantages:
- Breaks down into non-toxic byproducts (water and oxygen), making it environmentally friendly.
- Effective against a wide range of microorganisms, including spores.
- Considerations:
- It may be corrosive to certain materials, requiring compatibility checks.
- Vaporized hydrogen peroxide sterilization requires specialized equipment and monitoring.
-
Glutaraldehyde
- Form: A colorless liquid solution.
- Mechanism:
- Glutaraldehyde acts as a cross-linking agent, binding to proteins and nucleic acids, which inhibits their function and leads to cell death.
- Applications:
- Commonly used for sterilizing heat-sensitive instruments, such as endoscopes and surgical tools.
- Used in tissue preservation and as a disinfectant.
- Advantages:
- Effective against a wide range of pathogens, including bacteria and viruses.
- Rapid action (typically 10–30 minutes for sterilization).
- Considerations:
- It can be irritating to the skin, eyes, and respiratory system; proper ventilation and PPE are necessary.
- Requires rinsing after sterilization to remove residual chemical.
-
Peracetic Acid
- Form: Liquid, typically in an aqueous solution.
- Mechanism:
- Disrupts cellular functions by damaging proteins and nucleic acids.
- Acts rapidly and effectively at low concentrations.
- Applications:
- Used for sterilizing medical instruments and in the food processing industry.
- Effective for sterilizing surfaces and equipment.
- Advantages:
- Effective against a broad spectrum of microorganisms, including bacterial spores.
- Breaks down into non-toxic byproducts, similar to hydrogen peroxide.
- Considerations:
- It may be corrosive to some materials; compatibility testing is essential.
- Requires careful handling due to its strong oxidizing properties.
-
Chlorine Compounds
- Form: Typically used as diluted solutions (e.g., sodium hypochlorite).
- Mechanism:
- Chlorine compounds denature proteins and disrupt cell membranes, leading to microbial death.
- Applications:
- Widely used for disinfecting surfaces, water treatment, and laboratory settings.
- It is commonly used in hospitals for cleaning and disinfection.
- Advantages:
- Highly effective against many microorganisms, including bacteria, viruses, and fungi.
- Relatively low cost and widely available.
- Considerations:
- It can be corrosive to metals and some plastics; careful handling and material compatibility are essential.
- Strong odours and potential respiratory irritants require proper ventilation.
-
Iodine Compounds
- Form: Solutions such as povidone-iodine.
- Mechanism:
- Iodine alters protein structures and enzymatic functions, leading to cell death.
- Applications:
- Used as a skin antiseptic before surgeries and injections.
- Effective for disinfecting surfaces and in water treatment.
- Advantages:
- Broad antimicrobial activity, effective against bacteria, viruses, and fungi.
- Rapid action and relatively safe when used as directed.
- Considerations:
- It may cause skin irritation or allergic reactions in some individuals.
- Stains surfaces and fabrics, requiring careful application.
-
Alcohols (Ethanol and Isopropanol)
- Form: Liquid, typically used in concentrations of 60-90%.
- Mechanism:
- Denatures proteins and disrupts cell membranes, leading to cell lysis and death.
- Applications:
- It is commonly used for disinfecting skin, surfaces, and medical instruments.
- Effective for hand sanitization in healthcare settings.
- Advantages:
- Rapid action and ease of use.
- Evaporates quickly, leaving no residue.
- Considerations:
- Not effective against bacterial spores.
- Flammable must be stored and used with caution.
-
Formaldehyde
- Form: Available as gas or in aqueous solutions (formalin).
- Mechanism:
- Alkylates proteins and nucleic acids, leading to microbial death.
- Applications:
- Used for sterilizing medical equipment and preserving biological specimens.
- Advantages:
- Effective against a broad spectrum of pathogens, including spores.
- Considerations:
- Highly toxic and a known carcinogen, it requires strict safety measures and ventilation during use.
- Residual odors can be unpleasant and irritating.
Gaseous Sterilization
Gaseous sterilization involves using gaseous agents to eliminate all microbial life forms, including bacteria, viruses, fungi, and spores. It is particularly effective for heat-sensitive items that cannot withstand high temperatures.
Common Gaseous Sterilants
- Ethylene Oxide (EtO)
- Properties:
- A colorless, flammable gas with a faint odor.
- Effective at low temperatures (30-60°C).
- Mechanism:
- Alkylation: Ethylene oxide reacts with proteins and nucleic acids in microorganisms, disrupting essential cellular functions and leading to cell death.
- Applications:
- Widely used to sterilize surgical instruments, medical devices (like catheters), and some pharmaceuticals.
- Advantages:
- Excellent penetration abilities, allowing it to sterilize complex geometries and porous materials.
- Effective against a broad spectrum of microorganisms, including spores.
- Limitations:
- Highly toxic and flammable; requires careful handling and ventilation.
- Residual gas must be removed by aeration, which can take additional time.
- Properties:
- Hydrogen Peroxide Vapor
- Properties:
- A strong oxidizer that can be used in its vapor form for sterilization.
- Mechanism:
- Produces reactive oxygen species (free radicals) that damage proteins, lipids, and nucleic acids, resulting in microbial cell death.
- Applications:
- Commonly used to sterilize medical devices, laboratory equipment, and entire rooms (e.g., in hospitals).
- Advantages:
- Breaks down into non-toxic byproducts (water and oxygen), making it environmentally friendly.
- Fast-acting and effective against a broad range of microorganisms.
- Limitations:
- May cause corrosion or degradation of certain materials (e.g., some plastics).
- Requires specialized equipment for vaporization.
- Properties:
- Ozone (O₃)
- Properties:
- A powerful oxidizing gas with a characteristic smell.
- Mechanism:
- Ozone disrupts cellular processes by damaging the membranes and nucleic acids of microorganisms.
- Applications:
- Used for sterilizing food packaging materials, air, and water.
- Advantages:
- Highly effective against bacteria, viruses, and fungi.
- Decomposes into oxygen, leaving no harmful residues.
- Limitations:
- Requires careful control of concentration and exposure time to avoid material damage.
- Properties:
- Formaldehyde
- Properties:
- A colourless gas with a strong odour is commonly used in an aqueous solution (formalin).
- Mechanism:
- Alkylates proteins and nucleic acids, leading to cell death.
- Applications:
- Used for sterilizing laboratory equipment, biological specimens, and certain medical devices.
- Advantages:
- Effective against a broad range of pathogens, including spores.
- Limitations:
- Highly toxic and a known carcinogen, strict safety precautions are necessary.
- Unpleasant odor and potential for skin and respiratory irritation.
- Properties:
Procedure for Gaseous Sterilization
The process can vary based on the gaseous sterilization agent used but generally follows these steps:
1. Preparation
- Selection of Sterilant: Choose the appropriate gas based on the items to be sterilized, their material compatibility, and the specific microbial load.
- Safety Protocols: Review the safety guidelines for the selected gas, including required PPE and ventilation measures.
2. Container Selection
- Use Suitable Containers: Items should be placed in containers that can withstand exposure to the gas and allow for effective penetration. Materials should be non-reactive with the sterilant.
3. Loading the Sterilizer
- Arrange Items: Place items in the sterilization chamber with adequate spacing for uniform gas distribution and penetration.
4. Setting Parameters
- Conditions: Set specific conditions for the sterilization cycle:
- Ethylene Oxide: Typically 30-60°C, humidity around 40-80%, for several hours (4-6 hours), followed by aeration.
- Hydrogen Peroxide Vapor: Multi-phase cycles usually involve conditioning, exposure (20-60 minutes), and aeration.
- Ozone: Concentration levels and exposure times must be carefully controlled (generally 30-120 minutes).
5. Monitoring
- Ensure Proper Conditions: Monitor temperature, humidity, and gas concentration throughout the process to ensure effective sterilization.
6. Post-Sterilization Handling
- Aeration: After the cycle, especially for EtO, allow adequate aeration to remove residual gas.
- Inspect Items: Check for any signs of contamination or material degradation.
7. Documentation
- Record Keeping: Document the sterilization process, including the date, type of gas used, cycle parameters, and operator details for traceability and compliance.