
Introduction
-
Antiseptics and disinfectants are chemical agents used to control the growth of microorganisms and prevent infections.
-
They play a crucial role in healthcare, laboratories, and public health settings.
-
Antiseptics are applied on living tissues such as skin and mucous membranes to prevent infection.
-
Disinfectants are used on inanimate objects like laboratory benches, instruments, and hospital floors.
-
These agents help in reducing microbial load, but they do not always eliminate all microorganisms.
-
Their effectiveness depends on concentration, contact time, type of microorganism, and presence of organic matter.
-
Antiseptics and disinfectants act through various mechanisms such as cell membrane damage, protein denaturation, and oxidation.
-
Proper use is essential to ensure laboratory safety and infection control, especially for MLT students.
-
They are an important part of standard precautions and biosafety protocols in medical laboratories.
-
Understanding their uses and mode of action helps in selecting the right agent for a specific purpose.
Antiseptic
An antiseptic is a chemical agent applied to living tissues (such as skin and mucous membranes) to reduce the possibility of infection, sepsis, or putrefaction. Antiseptics work by killing or inhibiting the growth of microorganisms without causing significant harm to the host’s tissues.
Characteristics:
- Mechanism of Action: Antiseptics may disrupt cell membranes, denature proteins, or interfere with the metabolic processes of bacteria and other pathogens.
- Forms: Antiseptics can be found in various forms, including liquids (e.g., alcohol-based hand sanitisers), creams, and ointments (e.g., iodine solutions).
- Common Agents: Examples include alcohol (ethanol, isopropanol), iodine compounds (povidone-iodine), chlorhexidine, hydrogen peroxide, and triclosan.
Applications:
- Preoperative skin preparation
- Cleaning minor wounds and abrasions
- Hand hygiene in healthcare settings

Disinfectant
A disinfectant is a chemical substance that eliminates or significantly reduces pathogenic microorganisms on inanimate objects and surfaces. Disinfectants are stronger than antiseptics and are not intended for use on living tissues.
Characteristics:
- Mechanism of Action: Disinfectants generally act by disrupting cell walls, denaturing proteins, or damaging nucleic acids of microorganisms, leading to cell death.
- Forms: Commonly found as liquids, sprays, or wipes designed for surface cleaning.
- Common Agents: Examples include bleach (sodium hypochlorite), phenols, quaternary ammonium compounds, hydrogen peroxide (in higher concentrations), and formaldehyde.
Applications:
- Cleaning and sterilizing medical equipment and instruments
- Disinfecting surfaces in hospitals, laboratories, and public spaces
- Household cleaning of surfaces such as countertops and bathrooms
Types
Types of Antiseptics
- Alcohols (Ethanol, Isopropyl Alcohol)
- Uses: Commonly used for skin disinfection before injections and surgical procedures. Also used in hand sanitisers.
- Mode of Action:
- Denature proteins and disrupt cell membranes.
- Require a certain concentration (usually 60-90%) for optimal effectiveness, as higher concentrations evaporate too quickly to be effective.
- Iodine Compounds (Povidone-Iodine)
- Uses: Used for skin antisepsis before surgery and for treating minor wounds.
- Mode of Action:
- Releases free iodine, which penetrates the cell wall of microorganisms.
- Oxidizes and modifies proteins and nucleic acids, leading to microbial cell death.
- Chlorhexidine
- Uses: Utilized in pre-surgical skin antisepsis, oral rinses, and hand hygiene.
- Mode of Action:
- Disrupts bacterial cell membranes, causing leakage of intracellular components.
- Has residual antibacterial activity, meaning it continues to work after application.
- Hydrogen Peroxide
- Uses: Effective for wound cleaning and disinfecting surfaces in healthcare settings.
- Mode of Action:
- Generates reactive oxygen species (ROS) that damage proteins, lipids, and nucleic acids.
- High concentrations can be sporicidal, effective against spores.
- Benzalkonium Chloride (Quaternary Ammonium Compounds)
- Uses: Found in antiseptic wipes, surface disinfectants, and some hand sanitisers.
- Mode of Action:
- Disrupts cell membranes and denatures proteins, leading to cell lysis.
- It is effective against a broad range of bacteria but less effective against spores and some viruses.
Types of Disinfectants
- Chlorine Compounds (Sodium Hypochlorite)
- Uses: Widely used for water treatment and disinfecting surfaces in healthcare and food industry settings.
- Mode of Action:
- Releases free chlorine in solution, which oxidizes cellular components.
- Destroys cellular structures and functions, leading to cell death.
- Phenolic Compounds (Phenol, Cresol)
- Uses: Used as a disinfectant for surfaces in laboratories and healthcare facilities.
- Mode of Action:
- Denatures proteins and disrupts cell membranes, leading to cell lysis.
- Effective against bacteria, fungi, and some viruses.
- Aldehydes (Formaldehyde, Glutaraldehyde)
- Uses: Sterilization of medical instruments, preservation of biological specimens.
- Mode of Action:
- Cross-links proteins and nucleic acids, inactivating cellular functions.
- Effective against a wide range of pathogens, including spores.
- Peracetic Acid
- Uses: Disinfects medical equipment and is used in food processing.
- Mode of Action:
- Acts as a strong oxidizing agent, damaging proteins and lipids.
- Effective against bacteria, viruses, and fungi, including spores.
- Ozone
- Uses: Used for water treatment and air purification.
- Mode of Action:
- A strong oxidizer that reacts with organic materials, disrupting cellular functions.
- Effective against bacteria, viruses, and protozoa.
Uses
Uses of Antiseptics
-
Cleaning skin before venipuncture and blood collection
-
Preparation of donor site in blood banks
2. Hand Hygiene
-
Hand disinfection before and after specimen handling
-
Reduces laboratory-acquired infections
3. Wound and Surface Contact
-
Minor cuts in laboratory staff
-
Accidental exposure management
4. Patient Care Areas
-
Antiseptic mouthwashes and skin cleansers
Uses of Disinfectants
-
Cleaning benches, biosafety cabinets, and floors
-
Decontamination after sample spillage
2. Equipment and Instruments
-
Disinfection of centrifuges, pipettes, and glassware
-
Endoscopes and reusable lab instruments
3. Waste Management
-
Pre-treatment of biomedical waste
-
Disinfection of infectious materials
4. Water and Environmental Disinfection
-
Chlorination of laboratory water
-
Environmental sanitation
Mode of Action of Antiseptics and Disinfectants
Antiseptics and disinfectants kill or inhibit microorganisms through multiple biochemical and structural mechanisms.
1. Cell Wall and Cell Membrane Damage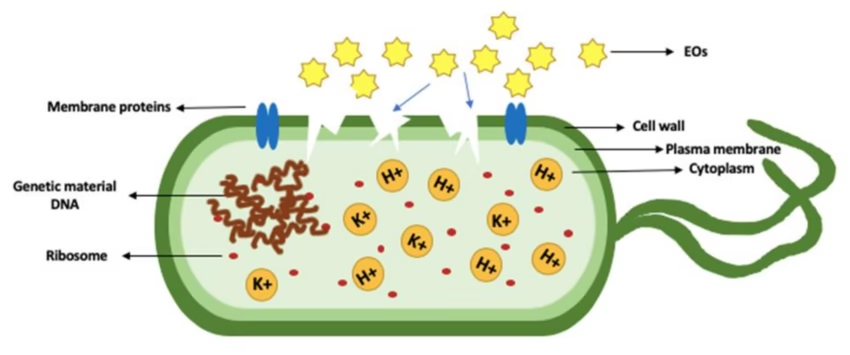
-
Disruption of lipid bilayer
-
Leakage of intracellular contents
-
Cell lysis and death
Examples: Phenols, alcohols, quaternary ammonium compounds
2. Protein Denaturation and Enzyme Inhibition
-
Coagulation of microbial proteins
-
Inactivation of metabolic enzymes
Examples: Alcohols, phenols, heavy metals
3. Oxidative Damage
-
Release of free oxygen radicals
-
Damage to DNA, proteins, and lipids
Examples: Hydrogen peroxide, potassium permanganate
4. Alkylation of Proteins and Nucleic Acids
-
Cross-linking of proteins and nucleic acids
-
Inhibits replication and cellular metabolism
Examples: Formaldehyde, glutaraldehyde
5. Interference with Cellular Metabolism
-
Inhibition of key metabolic pathways
-
Impaired ATP production
Examples: Halogens, biguanides
Spectrum of Activity
| Microorganism | Susceptibility |
|---|---|
| Gram-positive bacteria | High |
| Gram-negative bacteria | Moderate |
| Mycobacteria | Require higher concentration |
| Fungi | Variable |
| Enveloped viruses | Sensitive |
| Bacterial spores | Highly resistant |
Factors Affecting Efficacy
-
Concentration and contact time
-
Presence of organic matter (blood, pus)
-
Temperature and pH
-
Type of microorganism
-
Biofilm formation
Advantages and Disadvantages
Advantages of antiseptics:
- Skin Safety: Generally safe for use on living tissues.
- Infection Prevention effectively reduces the risk of infection in minor wounds and surgical sites.
- Rapid Action: Quickly reduces the microbial load on the skin.
Disadvantages of antiseptics:
- Skin Irritation: This can cause irritation or allergic reactions in some individuals.
- Limited Efficacy: Not effective against all pathogens, particularly spores.
- Resistance Risk: Overuse may contribute to microbial resistance.
Advantages of Disinfectants:
- Broad Spectrum: Highly effective against various pathogens, including bacteria, viruses, and fungi.
- Fast-Acting: Quickly eliminates microorganisms on surfaces.
- Long-Lasting: Often provides residual protection on surfaces.
Disadvantages of Disinfectants:
- Toxicity: Can be harmful to humans and animals if ingested or improperly handled.
- Surface Damage: May corrode or damage materials and equipment.
- Residue Issues: Potentially harmful residues if not rinsed off properly.
MCQs
Section A: Basics & Definitions
1. Antiseptics are chemical agents used on:
A. Inanimate objects
B. Living tissues
C. Surgical instruments only
D. Laboratory glassware
2. Disinfectants are best defined as agents that:
A. Sterilize all materials
B. Kill microorganisms on living tissues
C. Destroy microorganisms on inanimate objects
D. Stimulate immune response
3. Which of the following is the main difference between antiseptics and disinfectants?
A. Mode of action
B. Chemical structure
C. Toxicity and site of application
D. Spectrum of activity
4. An ideal antiseptic should be:
A. Highly toxic
B. Non-irritant and effective
C. Corrosive
D. Inflammable
5. Which term refers to killing of all forms of life including spores?
A. Disinfection
B. Antisepsis
C. Sterilization
D. Decontamination
Section B: Uses (Clinical & Laboratory)
6. Alcohol is commonly used in laboratories for:
A. Sterilizing culture media
B. Skin disinfection before venipuncture
C. Killing spores
D. Disinfecting surgical linen
7. Which disinfectant is commonly used for blood spillage in laboratories?
A. Phenol
B. Alcohol
C. Chlorine compounds
D. Chlorhexidine
8. Antiseptics are mainly used to:
A. Prevent infection
B. Treat systemic infections
C. Sterilize instruments
D. Destroy spores
9. Disinfectants are NOT recommended for:
A. Floors
B. Walls
C. Skin
D. Laboratory benches
10. In blood banks, skin antisepsis is essential to:
A. Prevent hemolysis
B. Prevent sample contamination
C. Increase blood flow
D. Reduce pain
Section C: Classification
11. Which of the following is a halogen disinfectant?
A. Phenol
B. Iodine
C. Chlorhexidine
D. Formaldehyde
12. Hydrogen peroxide belongs to which group?
A. Aldehydes
B. Phenolics
C. Oxidizing agents
D. Biguanides
13. Chlorhexidine is classified as:
A. Halogen
B. Aldehyde
C. Biguanide
D. Phenolic
14. Benzalkonium chloride is a:
A. Heavy metal
B. Alcohol
C. Quaternary ammonium compound
D. Aldehyde
15. Formaldehyde is mainly used as a:
A. Antiseptic
B. Sterilizing agent
C. High-level disinfectant
D. Detergent
Section D: Mode of Action
16. Alcohol kills bacteria mainly by:
A. Inhibiting DNA synthesis
B. Protein denaturation
C. Spore destruction
D. Cell wall synthesis inhibition
17. Phenolic compounds act primarily by:
A. Oxidation
B. Cell membrane damage
C. Alkylation of DNA
D. Enzyme activation
18. Which mechanism is used by hydrogen peroxide?
A. Protein precipitation
B. Free radical formation
C. Cell wall synthesis inhibition
D. pH alteration
19. Aldehydes act by:
A. Oxidizing proteins
B. Denaturing lipids
C. Cross-linking proteins and nucleic acids
D. Inhibiting ribosomes
20. Halogens exert antimicrobial action mainly by:
A. Reducing agents
B. Enzyme inhibition by oxidation
C. Protein synthesis stimulation
D. Cell wall thickening
Section E: Spectrum of Activity
21. Which organism is most resistant to disinfectants?
A. Gram-positive bacteria
B. Gram-negative bacteria
C. Enveloped viruses
D. Bacterial spores
22. Alcohols are ineffective against:
A. Mycobacteria
B. Fungi
C. Spores
D. Gram-positive bacteria
23. Which group is effective against Mycobacterium tuberculosis?
A. Alcohols
B. Aldehydes
C. QACs
D. Weak acids
24. Enveloped viruses are generally:
A. Highly resistant
B. Moderately sensitive
C. Highly sensitive
D. Completely unaffected
25. Which disinfectant has the widest spectrum?
A. Phenol
B. Alcohol
C. Glutaraldehyde
D. Chlorhexidine
Section F: Factors Affecting Activity
26. Presence of organic matter generally:
A. Enhances disinfectant activity
B. Has no effect
C. Reduces effectiveness
D. Sterilizes microbes
27. Increasing contact time usually:
A. Reduces activity
B. Has no effect
C. Increases effectiveness
D. Causes resistance
28. Biofilm formation leads to:
A. Increased susceptibility
B. Reduced disinfectant penetration
C. Faster killing
D. Complete sterilization
29. High temperature usually:
A. Decreases disinfectant activity
B. Inactivates antiseptics
C. Increases antimicrobial action
D. Has no role
30. pH affects disinfectant action by:
A. Changing microbial DNA
B. Altering chemical stability
C. Increasing spore count
D. Enhancing growth
Section G: Safety & Limitations
31. Which antiseptic may cause skin irritation on prolonged use?
A. Normal saline
B. Chlorhexidine
C. Alcohol
D. Distilled water
32. Phenol should be avoided in:
A. Adult skin
B. Neonates
C. Floors
D. Toilets
33. Excessive use of disinfectants may lead to:
A. Sterilization
B. Chemical burns
C. Increased immunity
D. Spore formation
34. Which disinfectant is corrosive to metals?
A. Alcohol
B. Chlorine
C. Chlorhexidine
D. Phenol
35. Antiseptics are usually used in:
A. High concentration
B. Undiluted form
C. Lower concentration
D. Solid form
Section H: Applied & Exam-Oriented
36. Best agent for hand hygiene in laboratories is:
A. Phenol
B. Alcohol-based rub
C. Formaldehyde
D. Glutaraldehyde
37. Which disinfectant is commonly used for OT fumigation?
A. Alcohol
B. Chlorhexidine
C. Formaldehyde
D. Iodine
38. Which agent acts by disrupting cell membrane lipids?
A. Alcohol
B. Phenol
C. QACs
D. All of the above
39. Which disinfectant is preferred for endoscopes?
A. Alcohol
B. Phenol
C. Glutaraldehyde
D. Hydrogen peroxide
40. Chlorine compounds are widely used because they are:
A. Expensive
B. Slow acting
C. Broad-spectrum
D. Non-toxic
Section I: True Concept Testing
41. Antiseptics can be used for sterilization.
A. True
B. False
42. Disinfectants are generally more toxic than antiseptics.
A. True
B. False
43. Alcohol acts better in presence of organic matter.
A. True
B. False
44. Most disinfectants are ineffective against spores.
A. True
B. False
45. Chlorhexidine has good activity against Gram-positive bacteria.
A. True
B. False
Section J: Mixed MCQs
46. Which agent acts by protein coagulation?
A. Alcohol
B. Aldehyde
C. Halogen
D. Oxidizing agent
47. Which is NOT a disinfectant?
A. Phenol
B. Chlorine
C. Alcohol hand rub
D. Formaldehyde
48. Which is safest for skin application?
A. Phenol
B. Formaldehyde
C. Chlorhexidine
D. Glutaraldehyde
49. Which disinfectant releases free oxygen?
A. Chlorine
B. Hydrogen peroxide
C. Phenol
D. Alcohol
50. Main purpose of antiseptics in laboratories is to:
A. Sterilize instruments
B. Reduce microbial load on skin
C. Destroy spores
D. Replace antibiotics
Answer
-
B
-
C
-
C
-
B
-
C
-
B
-
C
-
A
-
C
-
B
-
B
-
C
-
C
-
C
-
C
-
B
-
B
-
B
-
C
-
B
-
D
-
C
-
B
-
C
-
C
-
C
-
C
-
B
-
C
-
B
-
C
-
B
-
B
-
B
-
C
-
B
-
C
-
D
-
C
-
C
-
B
-
A
-
B
-
A
-
A
-
A
-
C
-
C
-
B
-
B