Introduction
-
Vitamins are organic micronutrients required in small amounts for normal metabolism, growth, and health.
They cannot be synthesized adequately by the body and must be obtained from the diet. -
They act mainly as coenzymes, antioxidants, and regulators of metabolic processes.
-
Vitamins are essential dietary compounds that support energy metabolism, tissue growth, immune function, and biochemical reactions.
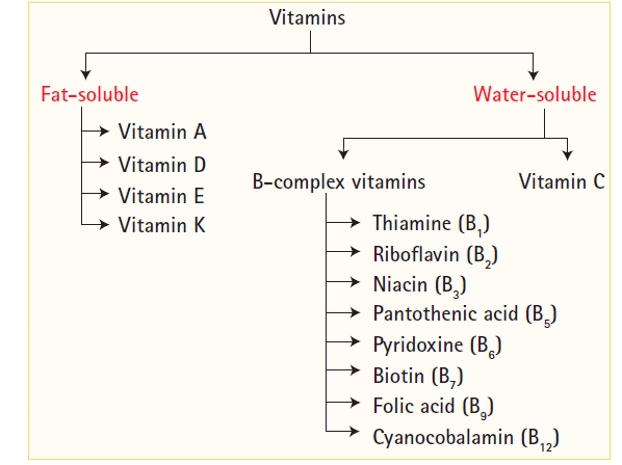
They are classified into:
-
-
Fat-soluble: A, D, E, K
-
Water-soluble: B-complex and C
-
Classification of vitamins
Fat-Soluble Vitamins
- Fat-soluble vitamins are a class of vitamins that dissolve in fats and oils and are absorbed along with dietary fat.
- These vitamins are stored in the body’s fatty tissues and liver and are used as needed by the body.
- Because they can be stored, fat-soluble vitamins are not needed as frequently as water-soluble vitamins, but excessive intake can lead to toxicity.
The four main fat-soluble vitamins are:
- Vitamin A (Retinoids)
- Vitamin D (Calciferols)
- Vitamin E (Tocopherols and Tocotrienols)
- Vitamin K (Phylloquinones and Menaquinones)
General Properties of Fat-Soluble Vitamins
- Solubility: Fat-soluble vitamins are soluble in fats and oils but not water.
- Absorption: They are absorbed in the small intestine along with dietary fats and require bile salts for optimal absorption.
- Storage: Fat-soluble vitamins are stored in the liver and fatty tissues, meaning they do not need to be consumed daily.
- Toxicity Risk: Due to their ability to accumulate in the body, consuming fat-soluble vitamins in excessive amounts can lead to toxicity (hypervitaminosis).
- Transport: These vitamins are transported in the bloodstream via lipoproteins or specific binding proteins since they are not water-soluble.
Vitamin A
Definition
Vitamin A is a fat-soluble vitamin essential for vision, epithelial differentiation, immunity, reproduction, and growth.
It exists as preformed vitamin A (retinoids) in animal foods and provitamin A carotenoids in plant sources.
Types and Forms
- Preformed Vitamin A (Retinoids): These are active forms found in animal products, including retinol, retinal, and retinoic acid.
- Retinol: The most usable form, converted to retinal (for vision) or retinoic acid (for cellular regulation).
- Retinoic acid: Critical for gene expression, growth, and development, including cell differentiation.
- Retinal: Key for the visual cycle in the retina, forming rhodopsin, which helps with night vision.
- Provitamin A (Carotenoids): Plant pigments that can be converted into Vitamin A in the body, such as beta-carotene, alpha-carotene, and beta-cryptoxanthin.
- Beta-carotene: A potent antioxidant and its conversion to retinol is carefully regulated to avoid toxicity from overconsumption.
Dietary Sources
Animal Sources (rich in retinol)
-
Liver (richest)
-
Fish liver oils
-
Egg yolk
-
Milk, butter, cheese
Plant Sources (β-carotene)
-
Carrot
-
Pumpkin
-
Sweet potato
-
Dark green leafy vegetables (spinach, amaranth, fenugreek)
-
Mango, papaya, apricot
Daily Requirements (RDA)
-
Men: 900 μg/day
-
Women: 700 μg/day
-
Pregnancy: 770 μg/day
-
Lactation: 1300 μg/day
-
Children: 300–600 μg/day
(Expressed as Retinol Activity Equivalents – RAE)
Absorption and Metabolism: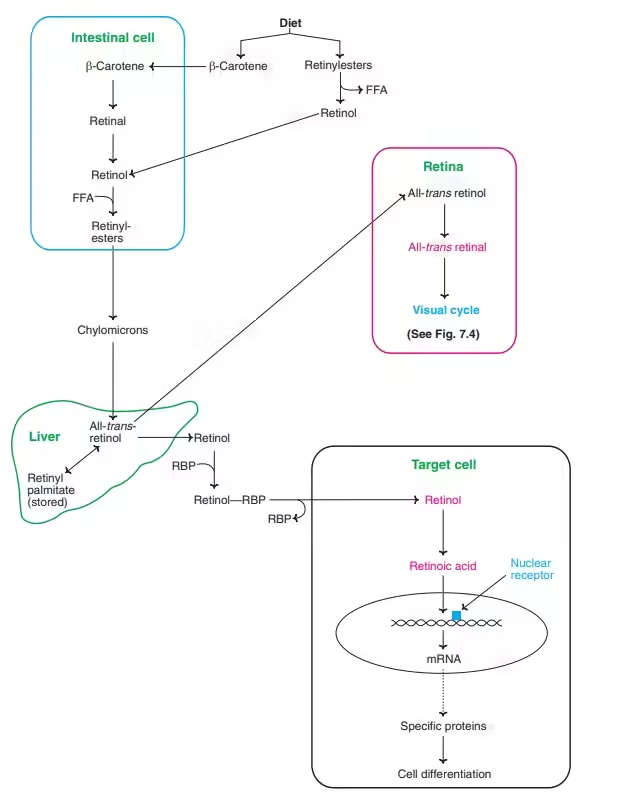
- Vitamin A is absorbed in the small intestine, requiring fat and bile salts for optimal uptake.
- Retinol is transported in the bloodstream by retinol-binding protein (RBP) and is stored primarily in the liver, where it can be released when needed.
- Carotenoids are absorbed similarly but are less efficiently converted to retinol in humans.
Wald’s visual cycle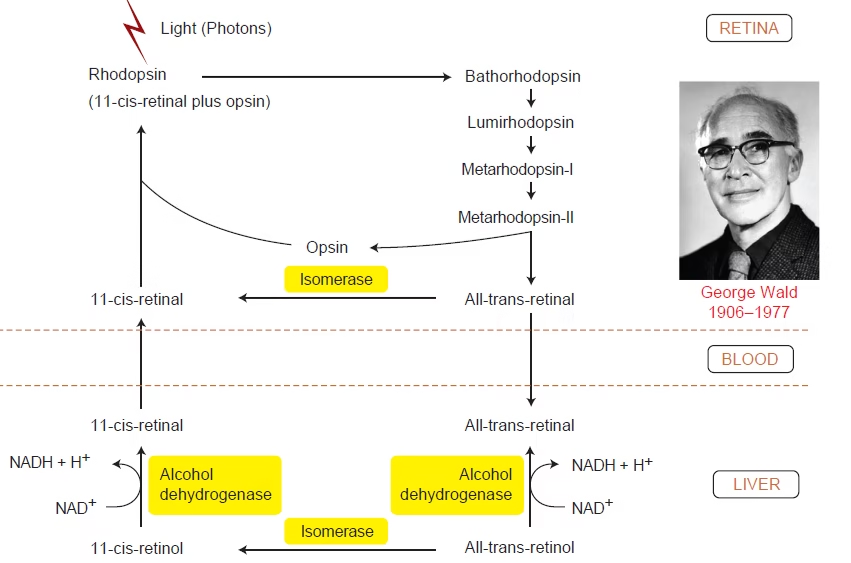
Wald’s visual cycle describes the photochemical and biochemical events in rod cells by which light is converted into a nerve impulse and the visual pigment is regenerated.
Rhodopsin = Opsin + 11-cis-retinal (vitamin A derivative)
Steps
- Dark state: Rhodopsin intact; rod cell depolarized.
- Light absorption: 11-cis-retinal → all-trans-retinal.
- Rhodopsin → metarhodopsin II.
- Metarhodopsin II activates transducin → phosphodiesterase.
- ↓ cGMP → closure of Na⁺ channels → hyperpolarization → visual signal.
- Bleaching: All-trans-retinal separates from opsin.
- Regeneration (RPE): All-trans-retinal → all-trans-retinol → 11-cis-retinal.
- 11-cis-retinal recombines with opsin → rhodopsin reformed.
Significance
- Essential for dim-light vision and dark adaptation.
Functions
- Vision:
- Vitamin A is critical for the synthesis of rhodopsin in the photoreceptor cells of the retina. Rhodopsin absorbs light and initiates the visual signal to the brain.
- Deficiency in Vitamin A deficiency hampers rhodopsin regeneration, leading to night blindness.
- Immune Function:
- Vitamin A supports the integrity of epithelial barriers (skin and mucous membranes), the body’s first defence against infections.
- It also regulates the immune system, enhancing the production and function of white blood cells like lymphocytes, which fight infections.
- Gene Expression and Cellular Growth:
- Retinoic acid regulates gene transcription by binding to nuclear receptors (RAR and RXR), which control gene expression in cell differentiation, embryonic development, and tissue repair.
- It is particularly important during fetal development and cell differentiation in tissues such as the skin, lungs, and gastrointestinal tract.
- Reproduction and Development:
- Vitamin A is vital for the development of sperm and the maintenance of pregnancy, influencing fetal growth and organ development.
Deficiency
1. Ocular Manifestations (Xerophthalmia – WHO Stages)
-
Night blindness (earliest sign)
-
Conjunctival xerosis (dry conjunctiva)
-
Bitot’s spots (foamy keratin patches)
-
Corneal xerosis
-
Keratomalacia → corneal softening, ulceration, blindness
-
Xerophthalmic fundus (late stage)
2. Skin and Epithelial Changes
-
Follicular hyperkeratosis (dry, rough, scaly skin)
-
Keratinization of respiratory, GIT, and genitourinary mucosa
-
↑ Risk of respiratory & gastrointestinal infections
3. Growth and Reproduction
-
Growth retardation in children
-
Impaired spermatogenesis
-
Fetal abnormalities during pregnancy
4. Immune Dysfunction
-
↓ Mucosal immunity
-
↑ susceptibility to infections (measles, diarrhea)
5. Hematological Effects
-
Microcytic anemia due to impaired iron mobilization
Toxicity (Hypervitaminosis A)
- Symptoms of Vitamin A toxicity include nausea, headaches, dizziness, blurred vision, and, in severe cases, liver damage and bone fractures.
- Teratogenic Effects: High doses of Vitamin A during pregnancy can cause severe birth defects.
Vitamin D
Definition
- Vitamin D is a fat-soluble vitamin functioning primarily as a hormone (calcitriol) involved in calcium–phosphate homeostasis.
- It is essential for bone mineralization, immune regulation, and cellular differentiation.
- It can be obtained from diet or synthesized in the skin by UV-B–mediated conversion of 7-dehydrocholesterol.
Types:
- Vitamin D2 (Ergocalciferol): Synthesized by plants and fungi when exposed to UV light.
- Vitamin D3 (Cholecalciferol): Produced in the skin when exposed to sunlight (UVB rays) or obtained from animal sources like fatty fish, liver, and fortified dairy products.
Dietary Sources
Animal Sources
-
Fish liver oils (richest)
-
Fatty fish: salmon, sardines, mackerel
-
Egg yolk
-
Liver
-
Butter
-
Fortified milk, cereals
Plant Sources
-
Mushrooms, fortified foods (D₂)
Endogenous Synthesis
-
UV-B sunlight converts 7-dehydrocholesterol → cholecalciferol (D₃) in the skin.
Daily Requirements (RDA)
-
Infants: 400 IU/day
-
Children & Adults: 600 IU/day
-
Elderly (>70 years): 800 IU/day
-
Pregnancy & lactation: 600 IU/day
Synthesis and Activation: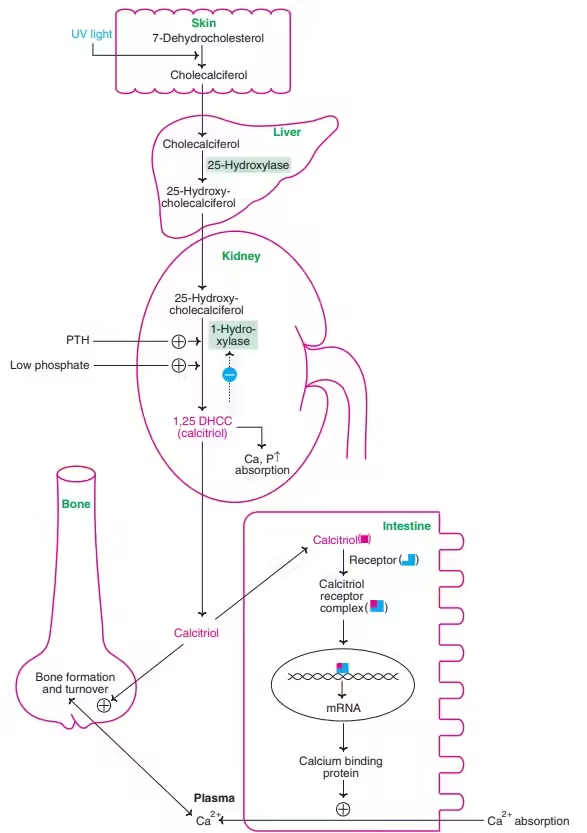
- Skin synthesis: UVB light converts 7-dehydrocholesterol in the skin to Vitamin D3.
- Both D2 and D3 are converted to their active form through two hydroxylation steps:
- The liver, where it is converted to 25-hydroxyvitamin D (calcidiol), is the main circulating form used to assess Vitamin D levels.
- In the kidneys, where it is converted to 1,25-dihydroxyvitamin D (calcitriol), the biologically active form.
Functions
- Calcium and Phosphorus Metabolism:
- Vitamin D stimulates the absorption of calcium and phosphate from the intestines.
- It promotes calcium reabsorption in the kidneys and the mobilization of calcium from bones to maintain normal blood calcium levels.
- Parathyroid hormone (PTH) works alongside Vitamin D to maintain calcium balance.
- Bone Health:
- Essential for bone mineralization, preventing softening of bones (rickets in children and osteomalacia in adults).
- Increases the activity of osteoblasts (bone-forming cells) and regulates bone resorption by osteoclasts.
- Immune Modulation:
- Vitamin D modulates innate and adaptive immune responses, enhancing the ability to fight respiratory infections.
- It helps reduce inflammation by suppressing pro-inflammatory cytokines.
- Cell Growth and Cancer Prevention:
- Regulates cellular proliferation and differentiation, with potential protective roles against certain cancers, such as colorectal, breast, and prostate cancer.
Deficiency
- Rickets: In children, this leads to soft, weak bones and skeletal deformities (bowed legs, thickened wrists, and ankles).
- Osteomalacia: In adults, deficiency causes softening of the bones, leading to bone pain and muscle weakness.
- Osteoporosis: Chronic deficiency contributes to decreased bone mass and increased risk of fractures in older adults.
Toxicity (Hypervitaminosis D):
- Over-supplementation of Vitamin D can lead to hypercalcemia (elevated blood calcium levels), causing nausea, vomiting, weakness, and kidney damage.
Vitamin E
Definition
Vitamin E is a fat-soluble antioxidant vitamin essential for protecting cell membranes from oxidative damage.
It refers to a group of compounds called tocopherols and tocotrienols, of which α-tocopherol is the most biologically active form.
Chemical Forms
Vitamin E exists in eight naturally occurring forms:
-
Tocopherols (α, β, γ, δ)
-
Tocotrienols (α, β, γ, δ)
α-Tocopherol is the major form found in human tissues and has the highest vitamin E activity.
Dietary Sources
Rich Sources
-
Wheat germ oil (richest natural source)
-
Sunflower oil, safflower oil
-
Almonds, peanuts
-
Hazelnuts, walnuts
Other Sources
-
Green leafy vegetables
-
Whole grains
-
Eggs
-
Avocado
-
Fortified cereals
Daily Requirements (RDA)
-
Adults: 15 mg/day of α-tocopherol
-
Pregnancy: 15 mg/day
-
Lactation: 19 mg/day
-
Requirements increase in people with high PUFA intake (due to increased oxidative stress)
Absorption and Transport:
-
Absorbed in the small intestine with dietary fats → requires bile salts.
-
Transported in chylomicrons → VLDL → LDL.
-
Stored in adipose tissue, liver, and muscle.
-
Hepatic α-tocopherol transfer protein (α-TTP) regulates plasma vitamin E levels.
Functions
- Antioxidant:
- Vitamin E neutralizes free radicals, particularly lipid peroxides, preventing oxidative damage to cell membranes, lipoproteins, and other cellular structures.
- Protects polyunsaturated fatty acids (PUFAs) in cell membranes from lipid peroxidation.
- Cardiovascular Protection:
- It may help reduce the risk of cardiovascular diseases by preventing oxidation of LDL cholesterol (oxidized LDL is more likely to lead to atherosclerosis).
- Some studies suggest Vitamin E reduces platelet aggregation and improves blood vessel function.
- Skin and Eye Health:
- Vitamin E helps protect skin cells from UV damage and reduces the risk of age-related macular degeneration (a leading cause of blindness in older adults).
- Immune Function:
- Vitamin E enhances T-cell function and may improve immune response, particularly in the elderly.
Deficiency
Causes
-
Fat malabsorption:
-
Cystic fibrosis
-
Cholestatic liver disease
-
Celiac disease
-
-
Premature infants (low stores)
-
Abetalipoproteinemia (defective chylomicron formation)
-
Low birthweight infants
Clinical Manifestations
1. Neuromuscular Symptoms
-
Peripheral neuropathy
-
Loss of deep tendon reflexes
-
Ataxia
-
Skeletal muscle weakness (myopathy)
-
Posterior column degeneration → impaired proprioception
2. Hematologic Effects
-
Hemolytic anemia (due to increased RBC membrane fragility)
-
Increased oxidative stress in RBCs
3. Retinopathy
-
Pigmented retinopathy (in severe deficiency)
4. Immune Dysfunction
-
Increased susceptibility to infections
Vitamin E Toxicity (Hypervitaminosis E)
Toxicity is rare but may occur with large doses (> 400–800 mg/day).
Symptoms
-
Increased risk of bleeding (interferes with vitamin K)
-
Gastrointestinal upset
-
Fatigue
-
Headache
-
Muscle weakness
Biochemical Effects
-
Prolonged prothrombin time
-
Reduced platelet aggregation
Vitamin K
Definition
- Vitamin K is a fat-soluble vitamin essential for the post-translational γ-carboxylation of glutamate residues in specific proteins, mainly blood clotting factors and bone-related proteins.
- It plays a crucial role in hemostasis, bone metabolism, and vascular health.
Chemical Forms
Vitamin K exists in three major forms:
1. Vitamin K₁ (Phylloquinone)
-
Found in green plants
-
Main dietary source
2. Vitamin K₂ (Menaquinones, MK-4 to MK-13)
-
Synthesized by intestinal bacteria
-
Found in fermented foods
3. Vitamin K₃ (Menadione)
-
Synthetic water-soluble form
-
Used therapeutically (not for neonates due to toxicity risk)
Dietary Sources
Rich Sources
-
Green leafy vegetables: spinach, kale, broccoli, cabbage
-
Brussels sprouts, lettuce, cauliflower
Other Sources
-
Soybean oil
-
Liver, meat, eggs
-
Fermented foods (natto – highest K₂ content)
-
Intestinal synthesis by gut microbiota (K₂ contributes significantly)
Daily Requirements
-
Men: 120 µg/day
-
Women: 90 µg/day
-
Higher needs in pregnancy/lactation and elderly
Absorption and Transport
-
Absorbed in the small intestine with bile salts and fat.
-
Transported via chylomicrons to the liver.
-
Stored in small amounts (rapid turnover → deficiency develops quickly).
Biochemical Functions of Vitamin K
1. γ-Carboxylation of Glutamate Residues (Primary Function)
Vitamin K acts as a coenzyme for γ-glutamyl carboxylase, converting glutamate (Glu) → γ-carboxyglutamate (Gla) residues.
This modification is essential for:
-
Coagulation factors: II (prothrombin), VII, IX, X
-
Anticoagulant proteins: Protein C, Protein S, Protein Z
-
Bone proteins: Osteocalcin, Matrix Gla protein (MGP)
2. Blood Coagulation
-
Gla residues bind calcium → allow clotting factors to attach to phospholipid surfaces → activation of coagulation cascade.
3. Bone Metabolism
-
Osteocalcin requires vitamin K for activation.
-
Essential for bone mineralization, prevention of osteoporosis.
4. Vascular Health
-
Matrix Gla protein (MGP) prevents vascular calcification.
-
Deficiency → arterial stiffness, calcification risk.
Vitamin K Cycle
-
Vitamin K is oxidized during Gla formation → regenerated by vitamin K epoxide reductase (VKOR).
-
Warfarin inhibits VKOR → inhibits recycling → anticoagulation.
Vitamin K Deficiency
Causes
-
Fat malabsorption (cholestasis, cystic fibrosis)
-
Prolonged broad-spectrum antibiotics (kill gut flora)
-
Newborns (low stores + sterile intestine)
-
Warfarin toxicity
-
Liver disease (reduced synthesis of clotting factors)
Clinical Manifestations
1. Hemorrhagic Disorders
-
Easy bruising
-
Mucosal bleeding
-
Petechiae, purpura
-
Hematuria
-
GI bleeding
-
Intracranial hemorrhage in severe cases
2. Prolonged Clotting Times
-
↑ PT (earliest and most sensitive)
-
↑ INR
-
APTT prolonged in severe deficiency
-
Normal platelet count
3. Newborn Bleeding (Hemorrhagic Disease of the Newborn)
-
Occurs in first few days of life
-
Due to low placental transfer + sterile gut + low vitamin K in breast milk
-
Prevented by 1 mg IM vitamin K at birth
Laboratory Findings in Deficiency
-
Prolonged PT (most sensitive test)
-
Normal bleeding time
-
Normal platelet count
-
PIVKA (Proteins Induced by Vitamin K Absence) ↑ — undercarboxylated clotting factors
Treatment
-
Vitamin K₁ (phytonadione): Oral or parenteral
-
For warfarin-induced bleeding:
-
Vitamin K + FFP or prothrombin complex concentrate
-
-
Neonates: prophylactic IM vitamin K at birth
Hypervitaminosis K
Rare, but menadione (synthetic K₃) can cause:
-
Hemolytic anemia
-
Hyperbilirubinemia
-
Kernicterus in infants
Natural K₁ and K₂ have very low toxicity.
Water-Soluble Vitamins
- Water-soluble vitamins are a group of vitamins that dissolve in water, are not stored in large amounts in the body, and therefore must be consumed regularly in the diet.
- They function mainly as coenzymes in numerous metabolic reactions and play essential roles in energy production, red blood cell formation, nervous system function, and antioxidant defense.
They include:
1. Vitamin C (Ascorbic Acid)
Important for collagen synthesis, antioxidant defense, iron absorption, and immunity.
2. B-Complex Vitamins
A group of vitamins that act primarily as coenzymes in carbohydrate, fat, and protein metabolism:
- Vitamin B1 (Thiamine)
- Vitamin B2 (Riboflavin)
- Vitamin B3 (Niacin)
-
Vitamin B5 (Pantothenic acid)
-
Vitamin B6 (Pyridoxine)
-
Vitamin B7 (Biotin)
-
Vitamin B9 (Folate/folic acid)
-
Vitamin B12 (Cobalamin)
General Properties of Water-Soluble Vitamins
- Solubility: They dissolve in water, making them easily absorbed in the intestines.
- Absorption: Water-soluble vitamins are absorbed directly into the bloodstream, primarily through the small intestine.
- Storage: Unlike fat-soluble vitamins, water-soluble vitamins are not stored in large quantities in the body. Excess is typically excreted in urine, so toxicity is rare but possible with excessive supplementation.
- Frequency of Intake: Because they are not stored, regular intake through diet is necessary to maintain adequate levels.
- Toxicity: Generally lower risk of toxicity due to their excretion in urine, although megadoses of certain vitamins can still cause adverse effects.
Vitamin B1
Definition
Vitamin B1 (Thiamine) is a water-soluble vitamin essential for carbohydrate metabolism and neural function.
Its active coenzyme form, Thiamine pyrophosphate (TPP), plays a key role in several oxidative decarboxylation and transketolase reactions.
Chemical Forms
-
Thiamine (free form)
-
Thiamine pyrophosphate (TPP) — active coenzyme
-
Thiamine triphosphate (TTP) — neuronal function
Dietary Sources
Rich Sources
-
Whole grains, cereals
-
Wheat germ
-
Legumes
-
Nuts and seeds
Animal sources
-
Pork (richest animal source)
-
Organ meats (liver, kidney)
-
Eggs, milk
Thiaminases present in:
-
Raw fish and tea → destroy thiamine
Daily Requirement (RDA)
-
Men: 1.2 mg/day
-
Women: 1.1 mg/day
-
Requirements increase in:
-
High carbohydrate intake
-
Pregnancy, lactation
-
Fever, hyperthyroidism
-
Alcoholism
-
Absorption & Storage
-
Absorbed in jejunum by active transport (low dose) and passive diffusion (high dose).
-
Stored in small amounts in liver → deficiency develops quickly (within 2–3 weeks).
Coenzyme Functions of Thiamine (TPP)
1. Oxidative Decarboxylation of α-keto acids
TPP acts as a coenzyme for:
-
Pyruvate dehydrogenase (Pyruvate → Acetyl-CoA)
-
α-Ketoglutarate dehydrogenase (TCA cycle)
-
Branched-chain α-ketoacid dehydrogenase (metabolism of leucine, isoleucine, valine)
2. Transketolase Reaction (HMP Shunt)
-
TPP is essential for transketolase, linking glycolysis and the pentose phosphate pathway.
-
Used as a diagnostic test: low RBC transketolase activity indicates deficiency.
3. Nerve Conduction
-
TPP needed for synthesis of acetylcholine.
-
Important for nerve impulse transmission and myelin maintenance.
Functions
-
Carbohydrate metabolism
-
ATP production
-
Pentose synthesis (nucleic acids)
-
Nervous system function
-
Neurotransmitter synthesis
Deficiency of Vitamin B1
Causes
-
Chronic alcoholism (↓ absorption + poor diet)
-
Malnutrition
-
Increased requirement (pregnancy, hyperthyroidism)
-
Dialysis
-
Diet high in polished rice
Clinical Manifestations
1. Dry Beriberi (Neurological type)
-
Peripheral neuropathy
-
Muscle wasting
-
Numbness, tingling
-
Absent reflexes
-
Foot drop
2. Wet Beriberi (Cardiac type)
-
High-output heart failure
-
Tachycardia
-
Edema
-
Cardiomegaly
3. Infantile Beriberi
-
Occurs in breast-fed infants of deficient mothers
-
Cardiac failure
-
Cyanosis
-
Convulsions
4. Wernicke’s Encephalopathy (acute thiamine deficiency in alcoholics)
-
Confusion
-
Ophthalmoplegia/nystagmus
-
Ataxia
Emergency — must give thiamine before glucose!
5. Korsakoff’s Psychosis (chronic stage)
-
Memory loss
-
Confabulation
-
Irreversible brain damage (mammillary body degeneration)
Laboratory Findings
-
Low RBC transketolase activity (most sensitive test)
-
Elevated pyruvate and lactate levels
-
Decreased urinary thiamine excretion
Vitamin B2
Definition
Vitamin B2 (Riboflavin) is a water-soluble vitamin essential for energy metabolism.
It functions mainly as the precursor of two important coenzymes:
-
FMN (Flavin mononucleotide)
-
FAD (Flavin adenine dinucleotide)
These coenzymes participate in oxidation–reduction reactions in numerous metabolic pathways.
Chemistry
-
Yellow, heat-stable pigment (destroyed by UV light).
-
Contains a ribitol side chain attached to a isoalloxazine ring.
-
Converted in the body to FMN and FAD.
Dietary Sources
Rich Sources
-
Milk and milk products (important in human diet)
-
Eggs
-
Liver, kidney
-
Green leafy vegetables
Other Sources
-
Whole grains, legumes
-
Brewer’s yeast
-
Almonds
Destroyed by
-
Exposure to sunlight (important for milk storage)
Daily Requirement (RDA)
-
Men: 1.3 mg/day
-
Women: 1.1 mg/day
-
Increased requirement in pregnancy, lactation, athletes, and hypermetabolic states.
Absorption, Transport & Storage
-
Absorbed in proximal small intestine.
-
Transported in plasma as riboflavin, FMN, or FAD bound to albumin.
-
Stored in liver, kidney, and heart in small amounts.
Coenzyme Functions
Vitamin B2 is vital for forming FMN and FAD, which act in:
1. Oxidation–Reduction Reactions
-
FAD/FMN serve as electron carriers in many enzymes (flavoproteins).
-
Examples:
-
Succinate dehydrogenase (TCA cycle & ETC Complex II)
-
Acyl-CoA dehydrogenase (fatty acid β-oxidation)
-
Glutathione reductase (antioxidant system)
-
2. Energy Production
-
FAD participates in the electron transport chain → ATP synthesis.
3. Metabolism of Other Vitamins
-
Converts Vitamin B6 to its active form (PLP).
-
Converts Folate to its active forms.
-
Essential for conversion of tryptophan → niacin.
4. Antioxidant Function
-
FAD is needed for glutathione reductase, which regenerates reduced glutathione (GSH).
Functions
-
Energy metabolism (carbohydrate, fat, protein metabolism)
-
Redox reactions via FAD/FMN
-
Maintenance of mucous membranes and skin
-
Antioxidant defense
-
Activation of other vitamins (B6, B9)
Deficiency of Vitamin B2 (Ariboflavinosis)
Causes
-
Inadequate dietary intake
-
Alcoholism
-
Chronic diarrhea
-
Malabsorption
-
Hyperthyroidism
-
Newborns receiving phototherapy (milk exposed to light)
Clinical Manifestations
1. Oropharyngeal Lesions (Classical signs)
-
Cheilosis: Fissuring and cracking of lips
-
Angular stomatitis: Cracks at corners of mouth
-
Glossitis: Magenta-colored, smooth tongue
-
Sore throat
2. Eye Changes
-
Photophobia
-
Itching and burning of eyes
-
Vascularization of cornea
3. Skin Lesions
-
Seborrheic dermatitis
-
Hyperemia and edema of mucous membranes
-
Nasolabial rash
4. Neurological
-
Peripheral neuropathy (rare)
5. Laboratory Marker
-
↓ activity of glutathione reductase in RBCs (FAD-dependent)
Vitamin B3
Definition
Vitamin B3 (Niacin) is a water-soluble vitamin that functions primarily as a precursor to two essential coenzymes:
-
NAD⁺ (Nicotinamide adenine dinucleotide)
-
NADP⁺ (Nicotinamide adenine dinucleotide phosphate)
These coenzymes are crucial for oxidation–reduction reactions, cellular energy production, DNA repair, and lipid metabolism.
Niacin exists as:
-
Nicotinic acid
-
Nicotinamide
Dietary Sources
Rich Sources
-
Meat, poultry
-
Fish (tuna, salmon)
-
Liver
Plant Sources
-
Whole grains, cereals
-
Legumes
-
Nuts (especially peanuts)
Special Note
-
Tryptophan → Niacin conversion occurs in the body
-
60 mg tryptophan ≈ 1 mg niacin equivalent
Foods like corn (maize) are low in niacin and tryptophan → predisposing to pellagra unless treated with alkali (nixtamalization).
Daily Requirement (RDA)
-
Men: 16 mg/day
-
Women: 14 mg/day
-
Pregnancy: 18 mg/day
-
Lactation: 17 mg/day
Requirements increase in:
-
Hypermetabolic states
-
High-protein or high-tryptophan diets
-
Chronic illness
Absorption, Transport & Storage
-
Absorbed in small intestine via simple diffusion.
-
Transported in blood as nicotinamide.
-
Stored in small amounts; excess eliminated in urine.
Biochemical Functions of Niacin
1. Component of NAD⁺ and NADP⁺
These coenzymes are essential for numerous redox reactions:
-
Glycolysis (GAPDH reaction)
-
TCA cycle
-
β-oxidation of fatty acids
-
Alcohol metabolism (ADH)
-
Pentose phosphate pathway
2. Energy Production
NAD⁺ plays a key role in electron transfer → ATP synthesis in mitochondria.
3. Fatty Acid & Cholesterol Metabolism
NADP⁺ is required for:
-
Fatty acid synthesis
-
Cholesterol synthesis
-
Desaturation reactions
4. DNA Repair & Gene Regulation
Niacin participates in:
-
PARP enzymes for DNA repair
-
Sirtuins (SIRT) involved in aging, metabolism, cell survival
5. Antioxidant Function
NADPH (derived from NADP⁺) is essential for:
-
Regeneration of reduced glutathione (GSH)
-
Reduction of methemoglobin
Deficiency of Niacin
Causes
-
Poor diet (especially maize-based)
-
Chronic alcoholism
-
Hartnup disease (↓ tryptophan absorption)
-
Carcinoid syndrome (↑ tryptophan → serotonin pathway)
-
Isoniazid therapy (interferes with B6 → ↓ niacin synthesis)
Clinical Features — Pellagra
The classical triad of 3 D’s:
1. Dermatitis
-
Photosensitive pigmented rash
-
Casal’s necklace (around the neck)
-
Hyperpigmentation on sun-exposed areas
2. Diarrhea
-
Inflammation of mucosa of GIT
-
Glossitis, stomatitis
-
Nausea, vomiting
3. Dementia
-
Depression, confusion
-
Memory loss
-
Hallucinations
-
Ultimately → neurological degeneration
If untreated → Death, the 4th “D”.
Laboratory Findings
-
Non-specific, often clinical
-
↓ NAD/NADP levels
-
Low tryptophan levels (Hartnup disease)
Treatment
-
Niacin or nicotinamide supplements: 100–300 mg/day
-
Treat underlying causes (alcoholism, malabsorption, poor diet)
-
High-protein diet to increase tryptophan intake
Toxicity of Niacin
High doses used to treat dyslipidemia (1–3 g/day) can cause:
1. Flushing
-
Most common
-
Due to prostaglandin release
-
Prevented by aspirin
2. Gastrointestinal upset
-
Nausea, vomiting, abdominal pain
3. Hepatotoxicity
-
Elevated liver enzymes
-
Can cause severe liver damage with sustained-release formulations
4. Hyperuricemia
-
May precipitate gout
5. Hyperglycemia
-
May worsen diabetes
Vitamin B5
Definition
Vitamin B5 (Pantothenic Acid) is a water-soluble vitamin essential for the synthesis of coenzyme A (CoA) and acyl carrier protein (ACP), which are required for numerous metabolic reactions involving carbohydrates, fats, and amino acids.
Chemical Nature
-
Composed of pantoic acid + β-alanine.
-
Stable in heat and acidic conditions but sensitive to alkali.
-
Widely distributed in foods → name “pantothenic” means “from everywhere”.
Dietary Sources
Pantothenic acid is ubiquitous in foods.
Rich Sources
-
Liver, kidney
-
Egg yolk
-
Meat, fish
-
Whole grains
-
Wheat germ
Other Sources
-
Legumes
-
Vegetables
-
Yeast
-
Milk
Because it is present in almost all foods, deficiency is rare.
Daily Requirement
-
Adults: 5 mg/day
-
Pregnancy: 6 mg/day
-
Lactation: 7 mg/day
Absorption, Transport & Storage
-
Absorbed in the jejunum by passive diffusion.
-
Present in blood as free pantothenate or as part of CoA.
-
Stored in small amounts in tissues; mainly in liver, adrenal gland, and kidneys.
Biochemical Functions
Pantothenic acid is essential for CoA and ACP synthesis, which take part in many key metabolic processes.
1. Component of Coenzyme A (CoA)
CoA is involved in:
-
TCA cycle (acetyl-CoA formation)
-
β-oxidation of fatty acids
-
Fatty acid synthesis
-
Cholesterol and steroid hormone synthesis
-
Acetylcholine synthesis
-
Heme synthesis
2. Component of Acyl Carrier Protein (ACP)
ACP is part of fatty acid synthase complex → essential for fatty acid elongation.
3. Metabolism of Macronutrients
CoA participates in:
-
Carbohydrate metabolism → oxidative decarboxylation
-
Lipid metabolism → synthesis and degradation
-
Amino acid metabolism → catabolism of leucine, valine, isoleucine
4. Synthesis of Important Molecules
-
Acetyl-CoA → central metabolic intermediate
-
Required for synthesis of:
-
Steroids
-
Bile acids
-
Ketone bodies
-
Neurotransmitters
-
Phospholipids
-
Deficiency of Vitamin B5
Deficiency is very rare because pantothenic acid is present in most foods.
Causes
-
Severe malnutrition
-
Chronic alcoholism
-
Prolonged use of certain medications
-
Genetic disorders affecting CoA metabolism
Clinical Manifestations
Burning Feet Syndrome (classical symptom):
-
Burning pain in feet
-
Numbness, tingling
-
Muscle cramps
Other symptoms:
-
Fatigue, irritability
-
GI disturbances (vomiting, abdominal cramps)
-
Sleep disturbances
-
Impaired wound healing
-
Hypotension
Vitamin B6
Definition
Vitamin B6 is a water-soluble vitamin required for amino acid metabolism, neurotransmitter synthesis, hemoglobin formation, and many enzymatic reactions.
Its active form is Pyridoxal-5-phosphate (PLP), a coenzyme in over 100 enzymatic reactions.
Chemical Forms
Vitamin B6 exists as three natural forms:
-
Pyridoxine (PN) – plant sources
-
Pyridoxal (PL)
-
Pyridoxamine (PM)
All are converted to the active coenzyme:
-
PLP (Pyridoxal phosphate)
-
PMP (Pyridoxamine phosphate)
Dietary Sources
Rich Sources
-
Meat, poultry, fish
-
Liver
-
Whole grains
Plant Sources
-
Nuts
-
Legumes
-
Bananas
-
Vegetables
Vitamin B6 is heat-labile—loss occurs during cooking.
Daily Requirement (RDA)
-
Adults: 1.3 mg/day
-
>50 years: 1.5 mg (women), 1.7 mg (men)
-
Pregnancy: 1.9 mg/day
-
Lactation: 2.0 mg/day
Requirements increase in:
-
High-protein diets
-
Oral contraceptive use
-
Alcoholism
Absorption, Transport & Storage
-
Absorbed in the jejunum by passive diffusion.
-
Converted in the liver to PLP.
-
PLP binds to albumin for transport.
-
Stored mainly in muscles (bound to glycogen phosphorylase).
Biochemical Functions
1. Amino Acid Metabolism
PLP is a coenzyme for:
-
Transamination (ALT, AST)
-
Decarboxylation → neurotransmitter synthesis
-
Transulfuration (cystathionine synthase)
-
Racemization
-
Deamination
2. Neurotransmitter Synthesis
PLP is required for synthesis of:
-
Serotonin (from tryptophan)
-
Dopamine, norepinephrine, epinephrine
-
GABA (from glutamate)
-
Histamine (from histidine)
3. Hemoglobin Synthesis
-
Required for heme formation (ALA synthase).
-
Deficiency → sideroblastic anemia.
4. Glycogenolysis
-
PLP is a cofactor for glycogen phosphorylase.
5. Homocysteine Metabolism
-
Helps convert homocysteine → cystathionine
-
Deficiency → ↑ homocysteine levels (CV risk).
6. Niacin Synthesis
-
Required for conversion of tryptophan → niacin.
7. Immune Function
-
Supports lymphocyte production and antibody formation.
Deficiency of Vitamin B6
Causes
-
Chronic alcoholism
-
Isoniazid therapy (INH forms inactive hydrazones with PLP)
-
Oral contraceptives
-
Renal dialysis
-
Malnutrition
-
Genetic disorders (e.g., pyridoxine-dependent epilepsy)
Clinical Manifestations
1. Neurological Symptoms
-
Peripheral neuropathy
-
Seizures (especially in infants)
-
Irritability
-
Depression (↓ serotonin)
2. Skin and Mucous Membrane Changes
-
Seborrheic dermatitis
-
Glossitis
-
Angular cheilosis
-
Stomatitis
3. Hematologic
-
Sideroblastic anemia (microcytic)
-
Impaired heme synthesis
4. Elevated Homocysteine
-
Risk of cardiovascular disease
5. In Infants
-
Convulsions
-
Irritability
-
Hypochromic anemia
Vitamin B7
Definition
Vitamin B7 (Biotin) is a water-soluble vitamin that functions as a coenzyme for carboxylase enzymes involved in fatty acid synthesis, amino acid metabolism, and gluconeogenesis.
It acts by transferring CO₂ in carboxylation reactions.
Chemical Nature
-
A sulfur-containing vitamin composed of a ureido ring fused with a thiophene ring.
-
Exists in free and protein-bound forms (biocytin).
-
Stable to heat, light, and oxidation.
Dietary Sources
Rich Sources
-
Egg yolk
-
Liver, kidney
-
Nuts (almonds, peanuts, walnuts)
-
Soybeans
-
Whole grains
Other Sources
-
Cauliflower
-
Mushrooms
-
Bananas
Special Note
-
Raw egg whites contain avidin, a protein that binds biotin strongly and prevents absorption → may cause deficiency in individuals consuming large amounts of raw eggs.
Microbial Synthesis
-
Intestinal bacteria synthesize biotin → contributes significantly to requirements.
Daily Requirement
-
Adults: 30 µg/day
-
Pregnancy: 30 µg/day
-
Lactation: 35 µg/day
Biotin requirement increases during pregnancy and in individuals on long-term antibiotics.
Absorption, Transport & Storage
-
Absorbed mainly in the jejunum via sodium-dependent transporter (SMVT).
-
Transported in plasma as free biotin or bound to proteins.
-
Stored in very small amounts; deficiency is uncommon due to dietary availability and bacterial synthesis.
Biochemical Functions of Biotin
Biotin serves as a coenzyme for four major carboxylase enzymes:
1. Acetyl-CoA Carboxylase
-
Converts acetyl-CoA → malonyl-CoA
-
First committed step in fatty acid synthesis
2. Pyruvate Carboxylase
-
Converts pyruvate → oxaloacetate
-
Important for:
-
Gluconeogenesis
-
Replenishing TCA cycle intermediates
-
3. Propionyl-CoA Carboxylase
-
Converts propionyl-CoA → methylmalonyl-CoA
-
Involved in metabolism of:
-
Odd-chain fatty acids
-
Valine, isoleucine, threonine, methionine
-
4. β-Methylcrotonyl-CoA Carboxylase
-
Involved in leucine catabolism
Additional Functions
-
Maintenance of skin, hair, and nails
-
Regulation of gene expression (biotinylation of histones)
Deficiency of Biotin
Deficiency is rare, but can occur.
Causes
-
Prolonged consumption of raw egg whites (avidin binds biotin)
-
Long-term antibiotic therapy (destroys gut flora)
-
Total parenteral nutrition (without supplementation)
-
Genetic defects in biotinidase or holocarboxylase synthetase (inherited metabolic disorders)
-
Severe malnutrition
-
Chronic alcohol use
Clinical Manifestations
1. Dermatological
-
Dermatitis (scaly, erythematous rash)
-
Alopecia (hair loss)
-
Brittle nails
2. Neurological
-
Depression
-
Lethargy
-
Hallucinations
-
Paraesthesia
-
Ataxia
-
Seizures (rare)
3. Metabolic
-
Lactic acidosis
-
Organic acidemia (in metabolic defects)
Characteristic Features
-
“Biotin deficiency = rash + alopecia + neurological symptoms”
Vitamin B9
Definition
- Vitamin B9 (Folate) is a water-soluble vitamin essential for one-carbon metabolism, DNA synthesis, cell division, and amino acid interconversions.
- It is especially crucial for rapidly dividing tissues such as bone marrow and embryonic tissues.
- The synthetic, more stable form used in supplements and food fortification is Folic Acid.
Chemical Nature
-
Folate consists of pteridine + para-aminobenzoic acid (PABA) + glutamate(s).
-
Natural folates are polyglutamates; absorbed form is monoglutamate.
Dietary Sources
Rich Sources
-
Green leafy vegetables (spinach, broccoli, lettuce)
-
Liver (very rich)
-
Legumes (beans, lentils)
-
Citrus fruits
-
Sprouts
Other Sources
-
Nuts, cereals, eggs
-
Fortified foods (bread, flour)
Destroyed by:
-
Heat (cooking)
-
Food processing
-
Prolonged storage
Daily Requirement (RDA)
-
Adults: 400 µg/day
-
Pregnancy: 600 µg/day
-
Lactation: 500 µg/day
Additional requirements:
-
Rapid growth (infancy, adolescence)
-
Hemolytic anemia
-
Chronic alcoholism
-
Malabsorption
Absorption, Transport & Storage
Absorption
-
Occurs mainly in jejunum.
-
Polyglutamate folates → converted to monoglutamate by folate conjugase.
-
Absorbed as folic acid or monoglutamate folate.
Transport
-
Circulates as 5-methyltetrahydrofolate (5-MTHF) bound to albumin.
Storage
-
Liver stores ~5–10 mg (deficiency develops in 2–4 months).
Metabolism of Folate (One-Carbon Metabolism)
Folate is reduced by dihydrofolate reductase (DHFR) to:
-
Dihydrofolate (DHF)
-
Tetrahydrofolate (THF) — active coenzyme form
THF carries one-carbon units (methyl, methylene, formyl) used in:
-
DNA synthesis (thymidylate and purine synthesis)
-
Amino acid metabolism
-
Methylation reactions
Biochemical Functions of Vitamin B9
1. DNA Synthesis
THF derivatives are required for:
-
Thymidylate (dTMP) synthesis
-
Purine (A, G) synthesis
Deficiency → impaired DNA synthesis → megaloblastic anemia.
2. Amino Acid Metabolism
-
Methionine synthesis (via 5-MTHF → requires B12)
-
Serine ↔ Glycine interconversion
-
Histidine metabolism
3. Methylation Reactions
Folate participates in the SAM (S-adenosylmethionine) cycle, important for:
-
DNA methylation
-
Neurotransmitter synthesis
-
Phospholipid synthesis
4. RBC Formation
Folate is essential for maturation and division of RBC precursors in bone marrow.
5. Fetal Development
Folate prevents:
-
Neural tube defects (NTDs): spina bifida, anencephaly
-
Congenital heart defects
-
Orofacial clefts
Deficiency of Vitamin B9
Causes
-
Poor diet (common)
-
Malabsorption (celiac disease, tropical sprue)
-
Chronic alcoholism
-
Pregnancy (increased requirement)
-
Anticonvulsants: phenytoin, valproate
-
Methotrexate (DHFR inhibitor)
-
Hemolytic anemia (high turnover)
Clinical Manifestations
1. Megaloblastic Anemia
-
Macrocytosis
-
Hypersegmented neutrophils
-
Weakness, pallor
-
Glossitis
2. Elevated Homocysteine
-
Due to impaired methionine synthesis →
-
↑ cardiovascular risk
3. No Neurological Symptoms
This differentiates folate deficiency from B12 deficiency, which causes neuropathy.
4. In Pregnancy
-
Neural tube defects (spina bifida, anencephaly)
-
Low birth weight
-
Placental abruption
-
Preterm birth
5. GI Symptoms
-
Diarrhea
-
Weight loss
Laboratory Findings
-
MCV ↑ (macrocytosis)
-
Hypersegmented neutrophils
-
Low serum folate
-
Low RBC folate (best indicator)
-
Elevated homocysteine
-
Normal methylmalonic acid (important distinction from B12 deficiency)
Vitamin B12
Definition
Vitamin B12 (Cobalamin) is a water-soluble vitamin essential for DNA synthesis, RBC formation, neurological function, and methylation reactions.
It contains cobalt at its core and is found only in animal foods.
It acts as a coenzyme in two major reactions:
-
Methionine synthase
-
Methylmalonyl-CoA mutase
Chemical Forms
Active coenzyme forms:
-
Methylcobalamin
-
Adenosylcobalamin
Other forms:
-
Hydroxocobalamin
-
Cyanocobalamin (supplement form)
Dietary Sources
Vitamin B12 is present only in animal-derived foods:
Rich Sources
-
Meat (beef, mutton)
-
Liver, kidney
-
Fish, shellfish
-
Eggs
-
Milk, cheese
Absent in plant foods
(Except fortified cereals or microbial fermentation)
Vegans are at high risk of deficiency.
Daily Requirement (RDA)
-
Adults: 2.4 µg/day
-
Pregnancy: 2.6 µg/day
-
Lactation: 2.8 µg/day
The body stores 2–5 mg, mainly in the liver → deficiency appears slowly (years).
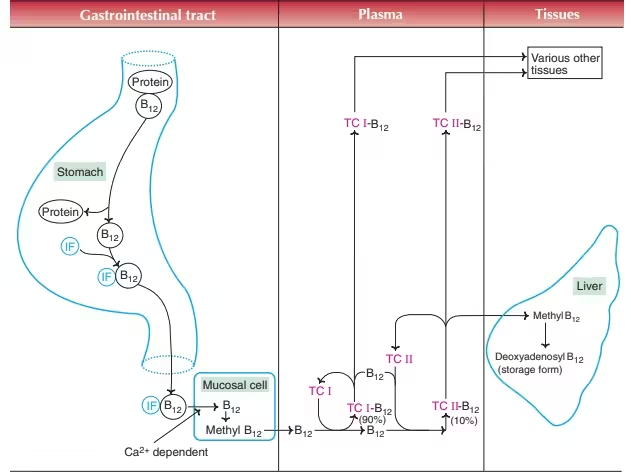
Absorption of Vitamin B12
Vitamin B12 absorption requires three proteins:
1. R-Binder (Haptocorrin) — from saliva
-
Binds B12 in stomach → protects it from acid.
2. Intrinsic Factor (IF) — from parietal cells
-
In the small intestine, pancreatic enzymes degrade R-binder → B12 binds to Intrinsic Factor.
-
B12–IF complex travels to ileum.
3. Cubilin Receptor (Ileal receptors)
-
B12–IF complex attaches to receptors → absorbed via endocytosis.
Transport
-
In blood, B12 binds to transcobalamin II → delivers B12 to tissues.
Storage
-
Liver stores large amounts (2–3 years reserve).
Biochemical Functions of Vitamin B12
1. Methionine Synthase Reaction
-
Converts homocysteine → methionine
-
Requires methylcobalamin
-
THF is regenerated from 5-MTHF
-
Necessary for:
-
DNA synthesis
-
RBC maturation
-
SAM (S-adenosylmethionine) formation (methyl donor)
-
Deficiency → “folate trap” → megaloblastic anemia
2. Methylmalonyl-CoA Mutase Reaction
-
Methylmalonyl-CoA → Succinyl-CoA
-
Requires adenosylcobalamin
-
Important for:
-
Odd-chain fatty acid metabolism
-
Myelin synthesis
-
Deficiency → ↑ methylmalonic acid → neurological damage
3. Other Roles
-
Myelin sheath integrity
-
Methylation of DNA, proteins, neurotransmitters
-
Energy production via Krebs cycle
Vitamin B12 Deficiency
1. Nutritional
-
Strict vegans
-
Malnutrition
-
Infants breastfed by deficient mothers
2. Malabsorption
-
Pernicious anemia (autoimmune destruction of parietal cells → no IF)
-
Atrophic gastritis
-
Gastrectomy
-
Bariatric surgery
-
Pancreatic insufficiency
-
Ileal diseases (Crohn’s, ileal resection)
-
Bacterial overgrowth
-
Tapeworm (Diphyllobothrium latum)
Clinical Manifestations
1. Megaloblastic (Macrocytic) Anemia
-
Pallor, fatigue
-
Weakness
-
Shortness of breath
-
Hypersegmented neutrophils
2. Neurological Manifestations
Due to impaired myelin synthesis:
-
Peripheral neuropathy
-
Paresthesias
-
Loss of vibration and position sense
-
Ataxia
-
Spasticity
-
Subacute Combined Degeneration of spinal cord (posterior columns + corticospinal tracts)
3. Neuropsychiatric Symptoms
-
Memory loss
-
Depression
-
Irritability
-
Dementia (in late cases)
4. Glossitis
-
Red, beefy tongue
5. Elevated Homocysteine & Methylmalonic Acid
-
Homocysteine ↑ (shared with folate deficiency)
-
Methylmalonic acid ↑ (specific for B12 deficiency)
Laboratory Findings
-
Macrocytic anemia (MCV ↑)
-
Low serum B12
-
High methylmalonic acid (MMA)
-
High homocysteine
-
Hypersegmented neutrophils
-
Positive anti–intrinsic factor antibodies (pernicious anemia)
-
Increased LDH & bilirubin due to ineffective erythropoiesis
Treatment
-
IM hydroxocobalamin or cyanocobalamin (for malabsorption/pernicious anemia)
-
Oral B12 for mild dietary deficiency
-
Lifelong therapy in pernicious anemia
-
Monitor for hypokalemia after treatment due to rapid RBC production
Toxicity
Very rare (water-soluble)
High doses may cause acne-like rash or hypersensitivity
Vitamin C
Definition
Vitamin C is a water-soluble, powerful antioxidant vitamin essential for collagen synthesis, iron absorption, immunity, neurotransmitter production, and wound healing.
Humans cannot synthesize vitamin C due to lack of the enzyme gulonolactone oxidase, so it must be obtained through diet.
Chemical Nature
-
Exists in two interchangeable forms:
-
Ascorbic acid (reduced form)
-
Dehydroascorbic acid (oxidized form)
-
-
Strong reducing agent → donates electrons easily.
-
Heat-labile and destroyed by cooking, storage, and alkali.
Dietary Sources
Richest Sources
-
Amla (Indian gooseberry)
-
Guava
-
Citrus fruits: orange, lemon, lime
Vegetables
-
Tomatoes, potatoes
-
Broccoli, cauliflower
-
Cabbage
-
Spinach, green peppers
Other
-
Strawberries, kiwi, papaya
-
Fresh fruits and salads
Daily Requirement (RDA)
-
Men: 90 mg/day
-
Women: 75 mg/day
-
Pregnancy: 85 mg/day
-
Lactation: 120 mg/day
-
Smokers: +35 mg/day (due to higher oxidative stress)
Needs increase during: infections, fever, stress, hyperthyroidism, burns, wound healing.
Absorption, Transport & Storage
Absorption
-
Occurs in jejunum via sodium-dependent vitamin C transporters (SVCT1 & SVCT2).
-
Dehydroascorbic acid absorbed via GLUT transporters.
Transport
-
Circulates freely in plasma.
-
High concentrations in:
-
Adrenal glands
-
Pituitary
-
Brain
-
Leukocytes
-
Lens of eye
-
Storage
-
Total body pool: ~1500 mg
-
Depletion occurs within weeks of low intake.
Excretion
-
Excess excreted as ascorbate or oxalate in urine.
Biochemical Functions
1. Collagen Synthesis (Most Important)
-
Cofactor for prolyl hydroxylase & lysyl hydroxylase.
-
Hydroxylation stabilizes collagen triple helix.
-
Deficiency → weak blood vessels, poor wound healing.
2. Antioxidant Function
-
Scavenges free radicals (ROS).
-
Regenerates Vitamin E (α-tocopherol) back to active form.
-
Protects lipids, proteins, and DNA from oxidative damage.
3. Iron Metabolism
-
Reduces Fe³⁺ → Fe²⁺ to enhance non-heme iron absorption.
-
Helps mobilize iron from ferritin.
4. Carnitine Synthesis
-
Cofactor in hydroxylation reactions forming carnitine, required for fatty acid transport into mitochondria.
-
Deficiency → fatigue, muscle weakness.
5. Neurotransmitter Synthesis
Required for:
-
Dopamine → Norepinephrine (via dopamine β-hydroxylase)
-
Serotonin synthesis
-
Controls mood regulation & stress response.
6. Peptide Hormone Activation
-
Needed for peptidyl-glycine amidation in hormones like:
-
Vasopressin
-
Oxytocin
-
CCK
-
7. Immune Functions
-
Enhances neutrophil chemotaxis and phagocytosis.
-
Protects leukocytes from oxidative damage.
-
Supports lymphocyte proliferation.
-
Improves wound healing.
8. Folate Metabolism
-
Reduces folic acid → tetrahydrofolate (THF).
9. Detoxification
-
Helps cytochrome P450 enzymes in detoxification & drug metabolism.
Deficiency of Vitamin C — Scurvy
Pathophysiology
-
Defective collagen synthesis → weak connective tissue.
Clinical Features
A. Skin & Hair
-
Perifollicular hemorrhages
-
Corkscrew hairs
-
Easy bruising
B. Oral Manifestations
-
Spongy, swollen gums
-
Bleeding gums
-
Loose teeth
-
Gingivitis
C. Musculoskeletal
-
Bone pain
-
Subperiosteal hemorrhage
-
Fragile bones in children
D. Wound Healing
-
Poor healing
-
Increased infection risk
E. Systemic
-
Fatigue, weakness
-
Depression
-
Anemia (↓ iron absorption + chronic blood loss)
Laboratory Findings
-
Low plasma ascorbic acid (<0.2 mg/dL)
-
Low leukocyte vitamin C level (most reliable)
-
Elevated bleeding time
-
Microcytic anemia
-
Low serum iron, ferritin
Treatment of Deficiency
-
Oral vitamin C: 100–500 mg/day
-
Severe deficiency: IV vitamin C
-
Rapid improvement in 1–2 weeks
Toxicity (Hypervitaminosis C)
Excess intake (>2 g/day) may cause:
Gastrointestinal
-
Diarrhea
-
Abdominal cramps
-
Nausea
Kidney
-
Increased risk of calcium oxalate kidney stones
Other
-
Interference with lab tests (false-high glucose readings)
-
Hemolysis in patients with G6PD deficiency
MCQs
- The active visual pigment in the retina is formed by opsin +:
A. Retinol
B. 11-cis-retinal
C. Retinoic acid
D. β-carotene - Bitot’s spots are characteristic of deficiency of:
A. Vitamin C
B. Vitamin A
C. Vitamin D
D. Vitamin K - The major storage site of vitamin A in the body is:
A. Kidney
B. Liver
C. Adipose tissue
D. Muscle - Retinoic acid exerts its action mainly via:
A. Membrane receptors
B. Nuclear receptors (RAR/RXR)
C. Enzyme inhibition
D. Antioxidant activity - Night blindness (nyctalopia) is earliest in deficiency of:
A. Vitamin D
B. Vitamin E
C. Vitamin A
D. Vitamin K - The provitamin A carotenoid with highest activity is:
A. Lycopene
B. β-carotene
C. Lutein
D. Zeaxanthin - Which vitamin is teratogenic in excessive amounts?
A. Vitamin C
B. Vitamin A (retinoids)
C. Vitamin K
D. Vitamin B2 - The transport protein for retinol in plasma is:
A. Transferrin
B. Retinol-binding protein (RBP)
C. Albumin only
D. Haptoglobin - The visual cycle converts 11-cis-retinal to:
A. 11-cis-retinol
B. All-trans-retinal
C. Retinoic acid
D. Retinyl ester - Bitot’s spots consist of:
A. Calcified plaques
B. Keratinized epithelium
C. Lipid deposits
D. Hemorrhage - The active hormonal form of vitamin D is:
A. Cholecalciferol
B. 25-hydroxyvitamin D (calcidiol)
C. 1,25-dihydroxyvitamin D (calcitriol)
D. Ergocalciferol - Rickets is due to deficiency of:
A. Vitamin A
B. Vitamin D
C. Vitamin E
D. Vitamin C - The skin precursor of vitamin D is:
A. Ergosterol
B. 7-dehydrocholesterol
C. Cholesterol sulfate
D. Lanosterol - First hydroxylation of vitamin D (to 25-OH D) occurs mainly in:
A. Kidney
B. Liver
C. Skin
D. Intestine - Renal 1α-hydroxylase activity is stimulated by:
A. High phosphate
B. Low PTH
C. PTH
D. Vitamin K - Vitamin D increases intestinal absorption of:
A. Iron
B. Calcium and phosphate
C. Zinc
D. Magnesium only - Osteomalacia in adults is due to defective:
A. Collagen synthesis
B. Bone mineralization
C. Vitamin C deficiency
D. Hematopoiesis - Hypervitaminosis D causes:
A. Hypocalcemia
B. Hypercalcemia
C. Scurvy-like features
D. Neuropathy - Ergocalciferol is:
A. Vitamin D3 from animal sources
B. Vitamin D2 from plant/fungal sources
C. Active form of vitamin D
D. A storage form in muscle - Rachitic rosary refers to enlargement of:
A. Costochondral junctions
B. Vertebral bodies
C. Knee epiphyses
D. Skull sutures - The most biologically active form of vitamin E is:
A. γ-tocopherol
B. δ-tocopherol
C. α-tocopherol
D. Tocotrienol - Major function of vitamin E is:
A. Coenzyme in dehydrogenases
B. Antioxidant protecting membranes
C. Hormone precursor
D. Calcium absorption - Deficiency of vitamin E commonly causes:
A. Hemolytic anemia and neurological deficits
B. Bleeding due to coagulopathy
C. Osteoporosis
D. Night blindness - Vitamin E protects membranes mainly by preventing:
A. Protein cross-linking
B. Lipid peroxidation
C. DNA strand breaks
D. Glycation - Which condition predisposes to vitamin E deficiency?
A. Fat malabsorption (cholestatic disease)
B. Excess carbohydrate intake
C. Vitamin K excess
D. High-fiber diet - Vitamin E works synergistically with:
A. Vitamin K
B. Vitamin C
C. Vitamin D
D. Vitamin B12 - High doses of vitamin E can antagonize which vitamin leading to bleeding risk?
A. Vitamin A
B. Vitamin K
C. Vitamin C
D. Vitamin D - The best dietary source of vitamin E is:
A. Citrus fruits
B. Wheat germ oil and nuts
C. Liver
D. Dairy milk - Neurological manifestations in vitamin E deficiency result from:
A. Demyelination
B. Hypocalcemia
C. Hyperkalemia
D. Thiamine deficiency - Plasma vitamin E is transported predominantly in:
A. HDL and LDL particles
B. Free form in plasma
C. Bound to albumin only
D. As vitamin E-RBP complex - Vitamin K is essential for γ-carboxylation of which amino acid residue?
A. Lysine
B. Glutamate (Glu → Gla)
C. Proline
D. Serine - Vitamin K is required for activation of which clotting factors?
A. I, V, VIII
B. II, VII, IX, X
C. III, V, VII
D. XI, XII - Warfarin acts by inhibiting:
A. Vitamin K epoxide reductase (VKOR)
B. 25-hydroxylase
C. Thrombin directly
D. Vitamin K absorption - Newborns are given vitamin K at birth primarily to prevent:
A. Rickets
B. Hemorrhagic disease of the newborn
C. Scurvy
D. Jaundice - Malabsorption of fat leads to deficiency of which vitamin among these?
A. Vitamin C only
B. Fat-soluble vitamins (A, D, E, K)
C. B-complex vitamins only
D. Vitamin B12 only - Which form of vitamin K is synthesized by gut bacteria?
A. Phylloquinone (K1) only
B. Menaquinone (K2)
C. Menadione (K3) only
D. None — all dietary - Prolonged antibiotic therapy may cause bleeding due to deficiency of:
A. Vitamin C
B. Vitamin K (reduced gut synthesis)
C. Vitamin B12
D. Vitamin A - The most sensitive routine lab test to detect early vitamin K deficiency is:
A. Bleeding time
B. Prothrombin time (PT)
C. Platelet count
D. Serum vitamin K level - Vitamin K is absorbed in the intestine with:
A. Water by passive diffusion
B. Bile salts and dietary fat
C. Active sodium-dependent transporter
D. Iron transporters - The synthetic vitamin K sometimes used clinically is:
A. Phylloquinone
B. Menadione (K3)
C. Ergocalciferol
D. Tocopherol - Vitamin C is required as a cofactor for which collagen-related enzymes?
A. Lysyl oxidase only
B. Prolyl and lysyl hydroxylases
C. Collagenase only
D. Transglutaminase - Humans cannot synthesize vitamin C because they lack:
A. Glucose-6-phosphatase
B. Gulonolactone oxidase
C. DOPA decarboxylase
D. Alcohol dehydrogenase - Classical triad of scurvy includes:
A. Dermatitis, diarrhea, dementia
B. Bleeding gums, perifollicular hemorrhages, poor wound healing
C. Night blindness, Bitot’s spots, xerosis
D. Megaloblastic anemia, glossitis, neuropathy - Vitamin C enhances absorption of dietary non-heme iron by:
A. Forming insoluble complexes
B. Reducing Fe³⁺ to Fe²⁺
C. Increasing gastric pH
D. Binding transferrin - Which is richest natural source of vitamin C?
A. Amla (Indian gooseberry)
B. Rice
C. Milk
D. Wheat - Vitamin C regenerates which other antioxidant vitamin?
A. Vitamin A
B. Vitamin E
C. Vitamin K
D. Vitamin D - Overdose of vitamin C can increase risk of:
A. Calcium oxalate kidney stones
B. Osteoporosis
C. Hemorrhagic stroke
D. Hyperkalemia - Vitamin C is important for synthesis of which neurotransmitter?
A. Serotonin
B. Norepinephrine (via dopamine β-hydroxylase)
C. Acetylcholine
D. GABA - Scurvy may present with characteristic hair change called:
A. Corkscrew hairs
B. Alopecia areata
C. Hirsutism
D. Pili torti - Vitamin C aids in formation of:
A. Collagen cross-links by hydroxylation of proline and lysine
B. γ-Carboxylation of glutamate
C. Methylation of homocysteine
D. Deamination of amino acids - Active coenzyme form of vitamin B1 is:
A. Thiamine monophosphate (TMP)
B. Thiamine pyrophosphate (TPP)
C. Thiamine triphosphate (TTP)
D. Thiamine aldehyde - TPP is a cofactor for which enzyme in carbohydrate metabolism?
A. Hexokinase
B. Pyruvate dehydrogenase (PDH)
C. Glucose-6-phosphatase
D. Lactase - A classic neuropsychiatric syndrome due to thiamine deficiency in alcoholics is:
A. Korsakoff-Wernicke complex
B. Pellagra
C. Scurvy
D. Subacute combined degeneration - Dry beriberi refers to predominant involvement of:
A. Heart
B. Peripheral nerves (neuropathy)
C. Liver
D. Skin - Wet beriberi is characterized by:
A. Cardiac failure and edema
B. Neuropathy only
C. Anemia only
D. Rash and photosensitivity - Which laboratory test is used to detect functional thiamine deficiency?
A. RBC transketolase activity (decreased)
B. Serum folate level
C. Serum B12 level
D. Serum iron - Thiamine deficiency leads to accumulation of:
A. Pyruvate and lactate
B. Acetoacetate
C. Citrate only
D. Glucose - Foods containing thiaminase (destroy thiamine) include:
A. Raw fish and certain plants
B. Cooked meat only
C. Citrus fruits
D. Dairy products - Recommended treatment for Wernicke’s encephalopathy includes:
A. IV glucose first
B. IV thiamine before glucose administration
C. Oral vitamin C only
D. High-dose niacin - Thiamine absorption primarily occurs in:
A. Stomach
B. Jejunum
C. Ileum
D. Colon - Active forms of riboflavin (B2) are:
A. NAD and NADP
B. FAD and FMN
C. CoA and ACP
D. PLP and PMP - Riboflavin deficiency (ariboflavinosis) commonly presents with:
A. Cheilosis, angular stomatitis, magenta tongue
B. Night blindness
C. Rickets
D. Hemolysis - Riboflavin is destroyed by:
A. Heat
B. UV light (sunlight)
C. Freezing
D. Drying - Riboflavin is a precursor for which enzyme involved in antioxidant defense?
A. Catalase
B. Glutathione reductase (FAD-dependent)
C. Superoxide dismutase
D. Peroxidase - Major dietary sources of riboflavin include:
A. Milk, eggs, liver
B. Sugary drinks
C. White rice only
D. Vegetable oil - Riboflavin deficiency may cause ocular symptoms including:
A. Photophobia and burning eyes
B. Night blindness only
C. Cataract immediately
D. Glaucoma - Riboflavin functions in which complex of electron transport chain?
A. Complex I (NADH dehydrogenase)
B. Complex II (succinate dehydrogenase, FAD-dependent)
C. Complex III
D. Complex IV - Riboflavin is required for conversion of vitamin B6 to:
A. PLP (pyridoxal phosphate)
B. TPP
C. NAD
D. THF - A diagnostic sign of riboflavin deficiency sometimes seen in infants receiving milk exposed to sunlight is:
A. Bright yellow urine
B. Magenta tongue in older children/adults
C. Jaundice only
D. Alopecia - Riboflavin supplementation can improve which skin condition?
A. Cheilosis and angular stomatitis
B. Psoriasis primarily
C. Acne vulgaris
D. Eczema only - Niacin (vitamin B3) is a precursor for:
A. FAD and FMN
B. NAD⁺ and NADP⁺
C. CoA
D. THF - Pellagra is classically described by the “three D’s”:
A. Dermatitis, diarrhea, dementia
B. Dermatitis, depression, dyspepsia
C. Diarrhea, dysphagia, dyspnea
D. Dementia, dysuria, deafness - Niacin can be synthesized in the body from:
A. Methionine
B. Tyrosine
C. Tryptophan
D. Lysine - A common adverse effect of pharmacologic high-dose niacin therapy is:
A. Hypotension
B. Facial flushing (due to prostaglandins)
C. Hair loss
D. Severe hypoglycemia - Hartnup disease predisposes to pellagra because of impaired absorption of:
A. Tryptophan
B. Tyrosine
C. Phenylalanine
D. Leucine - Niacin deficiency is uncommon in developed countries because:
A. Corn-based diets are rare and diets are varied
B. It is synthesized in skin by sunlight
C. It is abundantly stored in body fat
D. It is made by gut bacteria in large amounts - Niacin deficiency may be precipitated by which tuberculosis drug?
A. Rifampicin
B. Isoniazid (via B6 interactions)
C. Ethambutol
D. Streptomycin - Niacin is involved in which metabolic process?
A. One-carbon transfers
B. Redox reactions in catabolic pathways (dehydrogenases)
C. Carboxylation reactions
D. Glycosylation - Pharmacologic niacin is used to treat which lipid abnormality?
A. Low HDL
B. High LDL only
C. Low triglycerides only
D. Hypocholesterolemia - The niacin flush can be reduced by pre-treatment with:
A. Aspirin (prostaglandin inhibitor)
B. Vitamin C
C. Vitamin E
D. Antacids - Pantothenic acid (vitamin B5) is a constituent of:
A. Coenzyme A (CoA) and acyl carrier protein (ACP)
B. NADH only
C. FAD only
D. PLP only - Pantothenic acid deficiency classically causes:
A. Beriberi
B. Burning feet syndrome (rare)
C. Pellagra
D. Scurvy - Pantothenic acid is widely distributed in foods; its name means:
A. From plants
B. From everywhere (pantothen = everywhere)
C. From milk
D. From liver - CoA (derived from B5) is essential for:
A. Synthesis and oxidation of fatty acids, TCA cycle intermediate formation
B. DNA replication directly
C. Hydroxylation of proline
D. Formation of intrinsic factor - Pantothenic acid requirement increases in:
A. Low-fat diet
B. High metabolic/stress states (fever, trauma)
C. Vitamin K deficiency
D. Low protein intake - Which vitamin deficiency is most likely to cause burning feet in severe deficiency states?
A. B1
B. B5
C. B12
D. B2 - Pyridoxine (vitamin B6) active coenzyme is:
A. PLP (pyridoxal-5′-phosphate)
B. Thiamine pyrophosphate
C. FAD
D. NAD - PLP is required for which group of reactions?
A. Transamination, decarboxylation, racemization of amino acids
B. DNA replication
C. β-oxidation only
D. Glycolysis exclusively - Isoniazid therapy can induce deficiency of which vitamin?
A. B12
B. B6 (pyridoxine)
C. Vitamin C
D. Vitamin D - Vitamin B6 deficiency commonly causes which type of anemia?
A. Microcytic hypochromic anemia (due to impaired heme synthesis)
B. Macrocytic megaloblastic anemia
C. Hemolytic anemia
D. Aplastic anemia - Excessive chronic intake of pyridoxine can cause:
A. Hepatic failure
B. Sensory neuropathy (reversible on stopping)
C. Megaloblastic anemia
D. Scurvy - PLP is a coenzyme for which enzyme in heme synthesis?
A. ALA synthase (δ-aminolevulinic acid synthase)
B. Ferrochelatase
C. Uroporphyrinogen decarboxylase
D. Coproporphyrinogen oxidase - Vitamin B6 is required for conversion of tryptophan to:
A. Niacin (among other pathways)
B. Tyrosine
C. Histidine
D. Lysine - Major dietary sources of vitamin B6 include:
A. Meat, fish, whole grains, legumes
B. Sweets and soda
C. Refined rice only
D. Olive oil only - Pregnant women have slightly increased requirements for which B-vitamin?
A. B6 (pyridoxine) among others
B. B12 only
C. Vitamin K only
D. Vitamin E only - Folate (vitamin B9) active form used in one-carbon transfers is:
A. Tetrahydrofolate (THF) derivatives
B. FAD
C. NAD
D. PLP - Folate deficiency characteristically produces:
A. Microcytic anemia
B. Megaloblastic (macrocytic) anemia
C. Hemolytic anemia
D. Iron deficiency anemia - Folate is especially important in pregnancy to prevent:
A. Spina bifida and neural tube defects
B. Rickets
C. Scurvy
D. Pellagra - Serum homocysteine is elevated in folate deficiency because:
A. Methionine synthase activity is impaired
B. B12 is in excess
C. Iron absorption increases
D. Calcium decreases - Drugs that can cause folate deficiency include:
A. Methotrexate, trimethoprim, phenytoin
B. Vitamin C supplements only
C. Warfarin and aspirin only
D. Insulin and metformin - Best indicator of long-term folate status is:
A. Serum folate
B. RBC folate concentration
C. Urinary folate excretion
D. PT/INR - Folate is needed for de novo synthesis of:
A. Thymidylate (dTMP) and purines (A,G) for DNA synthesis
B. Hemoglobin directly
C. Vitamin B12
D. Vitamin C - Folate deficiency does NOT typically produce which feature (unlike B12 deficiency)?
A. Megaloblastic anemia
B. Neurological manifestations (e.g., subacute combined degeneration)
C. Elevated homocysteine
D. Glossitis - Folate supplements are recommended preconceptionally because:
A. They reduce neural tube defects when taken before and during early pregnancy
B. They cure rickets
C. They prevent scurvy
D. They increase vitamin D stores - Folate is converted to its active tetrahydro form by:
A. Dihydrofolate reductase (DHFR)
B. Alcohol dehydrogenase
C. Aldolase
D. Pyruvate kinase - Cobalamin (vitamin B12) contains which central metal ion?
A. Iron
B. Magnesium
C. Cobalt
D. Zinc - Two active coenzyme forms of B12 are:
A. Methylcobalamin and adenosylcobalamin
B. Cyanocobalamin only
C. Hydroxocobalamin only
D. Thiamine and riboflavin - Pernicious anemia is caused by autoimmune destruction of:
A. Parietal cells leading to lack of intrinsic factor (IF)
B. RBCs directly
C. Hepatocytes
D. Pancreatic islets - A specific biochemical marker elevated in B12 deficiency (but not folate deficiency) is:
A. Methylmalonic acid (MMA)
B. Homocysteine (only)
C. Ferritin
D. Serum iron - Neurological complication of B12 deficiency is:
A. Subacute combined degeneration of spinal cord (posterior columns + corticospinal tracts)
B. Peripheral facial palsy only
C. Pure motor neuron disease only
D. Wernicke’s encephalopathy - Major dietary sources of vitamin B12 include:
A. Meat, fish, dairy products, eggs (animal sources)
B. Green leafy vegetables only
C. Fruits only
D. Legumes only - B12 absorption in the ileum requires complexing with:
A. Haptocorrin only
B. Intrinsic factor (IF) produced by gastric parietal cells
C. Pepsin only
D. Bile salts only - Vegan diets predispose to deficiency of:
A. Vitamin C
B. Vitamin B12
C. Vitamin A only
D. Vitamin D only - Chronic metformin therapy predisposes to deficiency of:
A. Folate only
B. Vitamin B12 (reduced absorption)
C. Vitamin C only
D. Vitamin E only - The body stores of B12 are typically sufficient to prevent deficiency for:
A. A few days
B. Weeks
C. Years (often several years)
D. Never stored - Which of the following causes functional inactivation of B12 (oxidation) and can precipitate deficiency signs?
A. Nitrous oxide anesthesia (inactivates B12 by oxidizing cobalt)
B. Aspirin overdose
C. Insulin
D. Vitamin C large doses - Biotin (vitamin B7) acts as a coenzyme for:
A. Carboxylases (e.g., pyruvate carboxylase, acetyl-CoA carboxylase)
B. Dehydrogenases only
C. Kinases only
D. Phosphatases only - Biotin deficiency can be seen with chronic consumption of raw egg whites because of:
A. Avidin binding biotin and preventing absorption
B. Heat inactivation
C. Vitamin D interference
D. Excess protein - Clinical features of biotin deficiency include:
A. Dermatitis, alopecia, conjunctivitis, neurological symptoms
B. Bone pain and rickets only
C. Night blindness only
D. Hemolytic anemia only - Good dietary sources of biotin include:
A. Egg yolk, liver, yeast, nuts
B. White rice only
C. Olive oil only
D. Corn only - Which vitamin is most directly required for carnitine synthesis?
A. Vitamin C (ascorbic acid)
B. Vitamin B1
C. Vitamin B12
D. Vitamin D - Which vitamin is necessary for dopamine β-hydroxylase activity (dopamine → norepinephrine)?
A. Vitamin C
B. Vitamin B6
C. Vitamin K
D. Vitamin B12 - Which vitamin is required for activation of vitamin D (1α-hydroxylase stimulating PTH effect)?
A. Vitamin A
B. None — vitamin D activation regulated by PTH, phosphate, and kidney enzyme (not another vitamin)
C. Vitamin C
D. Vitamin K - Which vitamin deficiency causes pellagra-like dermatitis and diarrhea when tryptophan absorption is impaired?
A. Vitamin B3 (niacin)
B. Vitamin B1
C. Vitamin C
D. Vitamin B6 - Which vitamin is essential for formation of S-adenosylmethionine (SAM) cycle indirectly via methionine regeneration?
A. Vitamin B12 and folate (together)
B. Vitamin C alone
C. Vitamin E only
D. Vitamin D only - Which vitamin deficiency characteristically gives hypersegmented neutrophils on peripheral smear?
A. B12 and folate (megaloblastic anemias)
B. Vitamin C only
C. Vitamin K only
D. Vitamin A only - Which vitamin is most heat-labile and readily destroyed by cooking?
A. Vitamin C
B. Vitamin K
C. Vitamin D
D. Vitamin B12 - Which vitamin is necessary for hydroxylation of proline residues in collagen?
A. Vitamin C (cofactor for prolyl hydroxylase)
B. Vitamin D
C. Vitamin E
D. Vitamin A - Fat-soluble vitamins are absorbed primarily in:
A. Stomach
B. Small intestine with bile salts and dietary fat
C. Colon
D. Pancreas - Water-soluble vitamins are characteristically:
A. Stored in large amounts in adipose tissue
B. Not stored significantly and excreted in urine if excess
C. Toxic in small doses only
D. Always fat-soluble - Which vitamin deficiency is associated with hemorrhagic disease of the newborn?
A. Vitamin A
B. Vitamin K
C. Vitamin D
D. Vitamin B12 - Which vitamin is required for γ-carboxylation of osteocalcin in bone matrix?
A. Vitamin K
B. Vitamin D
C. Vitamin C
D. Vitamin A - Which vitamin is recommended as prophylaxis to prevent neural tube defects?
A. Vitamin B9 (folate)
B. Vitamin B12 only
C. Vitamin D only
D. Vitamin A only - Which vitamin is stored predominantly in the liver and adipose tissue and can cause toxicity if taken in excess?
A. Fat-soluble vitamins (A, D, E, K) — e.g., Vitamin A causes toxicity
B. Vitamin C only
C. Water-soluble B vitamins only
D. None - Which vitamin deficiency can present with peripheral neuropathy and ataxia resembling subacute combined degeneration?
A. Vitamin B12 deficiency
B. Vitamin C deficiency
C. Vitamin E deficiency only
D. Vitamin A deficiency - Which vitamin is important in carboxylation reactions as biotin cofactor?
A. Biotin (B7)
B. B12
C. B2
D. C - Which vitamin deficiency is implicated in increased susceptibility to measles complications in children and for which WHO recommends supplementation?
A. Vitamin A (reduces measles mortality)
B. Vitamin D
C. Vitamin E
D. Vitamin K - Which vitamin’s excess causes teratogenesis and spontaneous abortion risk if given in high doses during pregnancy?
A. Vitamin A (isotretinoin/retinoids are teratogenic)
B. Vitamin C
C. Vitamin K
D. Vitamin B2 - Which vitamin is essential for prothrombin synthesis?
A. Vitamin K (for γ-carboxylation of prothrombin)
B. Vitamin A
C. Vitamin C
D. Vitamin B6 - Which vitamin deficiency causes angular stomatitis, cheilosis, and magenta tongue?
A. Riboflavin (B2) deficiency
B. B12 deficiency only
C. Vitamin C deficiency only
D. Vitamin A deficiency only - The American RDA for vitamin C for adult men is approximately:
A. 90 mg/day
B. 5 mg/day
C. 1000 mg/day
D. 10 mg/day - The active coenzyme form of folate that donates one-carbon units is:
A. 5-methyltetrahydrofolate (5-MTHF) and other THF derivatives
B. NADH
C. PLP
D. Biotin - Which vitamin deficiency can cause microcytic anemia due to impaired heme synthesis?
A. Vitamin B6 (PLP deficiency) can cause microcytic anemia
B. B12 only
C. Folate only
D. Vitamin C only - Which vitamin is required for the regeneration of reduced glutathione via glutathione reductase (FAD-dependent)?
A. Riboflavin (B2) via FAD cofactor
B. Vitamin C only
C. Vitamin A only
D. Vitamin K only - Which vitamin is an essential cofactor for pyruvate carboxylase (gluconeogenesis) as biotin-dependent enzyme?
A. Biotin (B7)
B. B1
C. B12
D. B9 - Which vitamin supplementation is recommended for pregnant women to prevent neural tube defects?
A. Folic acid (400–800 µg preconceptionally)
B. Vitamin A at high doses
C. Vitamin D only
D. Vitamin E only - Which vitamin deficiency is linked to cheilosis, stomatitis and seborrheic dermatitis and may cause anemia?
A. Riboflavin (B2) deficiency
B. Niacin deficiency only
C. B12 deficiency only
D. Vitamin D deficiency only - Which vitamin deficiency can cause infantile beriberi when mother is deficient and breastfeeding?
A. Thiamine (B1) deficiency
B. Vitamin C deficiency
C. B12 deficiency only
D. Riboflavin deficiency - Which vitamin is essential for the formation of coenzyme A and therefore central to acetyl-CoA formation?
A. Pantothenic acid (B5)
B. Vitamin C
C. Vitamin K
D. Riboflavin - Which vitamin is most critical in hydroxylation reactions of cholesterol side chain in the pathway of steroidogenesis?
A. Vitamin C (cosubstrate in certain hydroxylases) — though steroidogenesis mainly requires cytochrome P450 enzymes with NADPH, vitamin C supports some hydroxylation reactions.
B. Vitamin D
C. Vitamin K
D. Vitamin A
Answer Key (1–150)
1-B
2-B
3-B
4-B
5-C
6-B
7-B
8-B
9-B
10-B
11-C
12-B
13-B
14-B
15-C
16-B
17-B
18-B
19-B
20-A
21-C
22-B
23-A
24-B
25-A
26-B
27-B
28-B
29-A
30-A
31-B
32-B
33-A
34-B
35-B
36-B
37-B
38-B
39-B
40-B
41-B
42-B
43-B
44-B
45-A
46-B
47-A
48-B
49-A
50-A
51-B
52-B
53-A
54-B
55-A
56-A
57-A
58-A
59-B
60-B
61-B
62-A
63-B
64-B
65-A
66-A
67-B
68-A
69-B
70-A
71-B
72-A
73-C
74-B
75-A
76-A
77-B
78-B
79-A
80-A
81-A
82-B
83-B
84-A
85-B
86-B
87-A
88-A
89-B
90-A
91-B
92-A
93-A
94-A
95-A
96-A
97-B
98-A
99-A
100-A
101-B
102-A
103-B
104-A
105-A
106-C
107-A
108-A
109-A
110-A
111-A
112-B
113-B
114-B
115-C
116-A
117-A
118-A
119-A
120-A
121-A
122-A
123-B
124-A
125-A
126-A
127-A
128-A
129-B
130-B
131-B
132-A
133-A
134-A
135-A
136-A
137-A
138-A
139-A
140-A
141-A
142-A
143-A
144-A
145-A
146-A
147-A
148-A
149-A
150-A