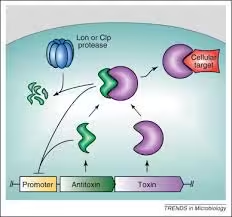
- The toxin-antitoxin (TA) system is a stress-response system used by bacteria.
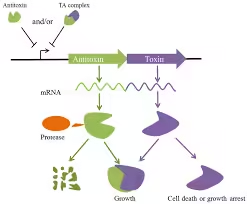
- It comprises two components: the toxin, which can inhibit cellular functions or lead to cell death, and the antitoxin, which counteracts the toxin under normal conditions.
- These systems play roles in bacterial persistence, biofilm formation, stress resistance, and pathogenicity.
Mechanism of Toxin-Antitoxin Systems
- Types of TA Systems:
- Type I: The antitoxin is a small RNA molecule inhibiting toxin mRNA translation.
- Type II: Both toxin and antitoxin are proteins. The antitoxin directly binds to the toxin to inhibit it.
- Type III, IV, V, and VI include mechanisms such as RNA binding, enzymatic degradation of the toxin, and binding to different cellular targets.
- Functionality in Persistence:
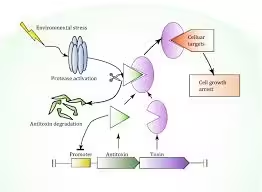
- TA systems allow bacterial populations to enter a state of dormancy (or persistence) under stress, such as during antibiotic treatment, helping the bacteria survive.
- Mechanism: Stress triggers proteases to degrade the antitoxin, allowing the toxin to act and inhibit essential processes, leading to a dormant state in some cells.
Toxin-Antitoxin Assay Techniques
- Growth Inhibition Assay:
- Objective: Measures the toxin’s ability to inhibit bacterial growth.
- Procedure: The toxin gene is expressed in a controlled system (e.g., under an inducible promoter), and bacterial growth is measured in the presence or absence of the antitoxin.
- Outcome: If the toxin is active, growth inhibition occurs. Normal growth resumes when the antitoxin is co-expressed, confirming the system’s function.
- Fluorescence and Reporter-Based Assays:
- Objective: Visualize the interaction between toxin and antitoxin, assess cellular localization, and measure the toxin’s effects on bacterial cells.
- Procedure: GFP (green fluorescent protein) or other reporter proteins are fused to the toxin and/or antitoxin, and fluorescence microscopy or flow cytometry tracks expression, binding, and cellular effects.
- Outcome: Observing reduced fluorescence in specific cellular regions can indicate where the toxin and antitoxin interact or how the toxin acts within the cell.
- Cell Viability Assays:
- Objective: Measure cell survival following toxin exposure.
- Procedure: Bacterial cultures expressing toxin, with or without antitoxin, are subjected to a viability assay like MTT, where metabolically active cells convert a tetrazolium dye into a colored product.
- Outcome: Reduced color formation indicates that the toxin is inhibiting cell survival; if color remains, it suggests antitoxin neutralization of the toxin.
- Gene Knockout and Mutagenesis Studies:
- Objective: Identify the specific role of TA genes in pathogenicity or persistence.
- Procedure: The toxin or antitoxin genes are deleted or mutated, and the bacteria are subjected to environmental stresses (e.g., antibiotics).
- Outcome: Observing decreased survival or persistence in knockout strains versus wild types suggests the TA system’s role in stress response.
- Protease Assays:
- Objective: Examine the regulation of antitoxin degradation by stress-induced proteases.
- Procedure: Bacterial cultures are stressed (e.g., heat or nutrient deprivation), and protease activity is measured to determine how the antitoxin is degraded, freeing the toxin.
- Outcome: Increased protease activity in stressed cells confirms the mechanism by which TA systems are activated in response to environmental cues.
Applications in Research and Medicine
- Antibiotic Development: Understanding TA systems helps identify targets for drugs that can inhibit the toxin-antitoxin interaction or exploit the system to kill bacteria.
- Control of Persistent Infections: Since TA systems contribute to bacterial persistence, targeting these systems could help eliminate chronic infections.
- Biofilm Formation: Many TA systems regulate biofilm formation, a key virulence factor, so these assays help understand biofilm-associated infections.
Pathogenicity Tests:
Pathogenicity tests are essential for understanding how microorganisms cause disease. These tests assess the ability of pathogens to adhere, invade, and damage host tissues and measure the host’s immune response to infection.
Mechanisms of Pathogenicity
- Adhesion: Pathogens must adhere to host cells, often mediated by surface proteins, pili, or fimbriae.
- Invasion: After adhesion, pathogens may invade host cells, allowing them to evade the immune response and establish infection.
- Toxin Production: Pathogens release toxins that damage host cells and facilitate infection.
- Immune Evasion: Pathogens, such as hiding within host cells or expressing surface molecules that mimic host proteins, deploy mechanisms to avoid or subvert the immune system.
Types of Pathogenicity Tests and Procedures
- In Vivo Pathogenicity Tests:
- Objective: Assess a living organism’s virulence, disease progression, and immune response.
- Procedure: Animals are inoculated with a pathogen, and symptoms, bacterial load, and survival rates are tracked. Depending on the pathogen, mice, rabbits, and non-human primates are commonly used.
- Outcome: Higher pathogenicity correlates with rapid progression of symptoms, high bacterial counts in tissues, and high mortality rates. Comparing mutant and wild-type strains in animals also identifies genes critical for infection.
- LD50 and ID50 Assays:
- Objective: Quantify virulence by determining the lethal (LD50) or infectious dose (ID50).
- Procedure: Different pathogen doses are administered, and survival or infection rate is measured.
- Outcome: Lower LD50 or ID50 values indicate higher virulence.
- In Vitro Cell Culture Assays:
- Objective: Assess the pathogen’s ability to adhere to, invade, and kill host cells.
- Procedure: Pathogens are introduced to cultured host cells (e.g., epithelial or macrophage cells). Microscopy and staining techniques (e.g., crystal violet for adhesion) assess bacterial attachment or invasion.
- Outcome: High adhesion or invasion rates indicate pathogenic potential and cytopathic effects (cellular changes like lysis or detachment) show pathogenic damage.
- Adhesion and Invasion Assays:
- Objective: Quantify the pathogen’s ability to attach to and enter host cells.
- Procedure: Bacterial cells are introduced to host cells, and non-adherent bacteria are washed away. For invasion assays, host cells are lysed after incubation, and intracellular bacteria are plated to count viable bacteria.
- Outcome: Higher counts indicate stronger adherence or invasion capabilities, contributing to pathogenicity.
- Toxin Assays:
- Objective: Measure the effect of bacterial toxins on host cells, often using cytotoxicity assays.
- Procedure: Toxins are purified and exposed to target cells (e.g., red blood cells for hemolytic toxins), and cell death or lysis is monitored.
- Outcome: Observing cell damage or death indicates the toxin’s potency. Hemolytic assays, for instance, measure hemolysin production by observing red blood cell lysis.
- Gene Knockout and Mutagenesis Studies:
- Objective: Determine the function of specific virulence genes.
- Procedure: Mutant strains lacking certain virulence genes are created, and pathogenicity is compared with the wild-type strain.
- Outcome: Reduced virulence in mutants suggests that the knocked-out gene is essential for infection, identifying it as a possible drug target.
- Host Immune Response Assays:
- Objective: Evaluate the host immune response to infection.
- Procedure: Cytokine levels in blood or tissue samples from infected animals are measured using ELISA, flow cytometry, or qPCR.
- Outcome: Elevated cytokine levels indicate inflammation or immune activation, while lack of response may indicate immune evasion.
- In Vivo Imaging (e.g., Bioluminescence Imaging):
- Objective: Track infection in real-time in living hosts.
- Procedure: Pathogens engineered to express bioluminescent proteins are used to infect animals, and specialized cameras capture bioluminescence over time.
- Outcome: Real-time visualization of infection helps monitor bacterial spread, tissue tropism, and response to treatment.
Applications of Pathogenicity Tests
- Vaccine Development: Pathogenicity tests help identify antigens and virulence factors crucial for infection, which are ideal candidates for vaccine development.
- Antimicrobial Testing: By assessing how pathogens interact with host cells, these tests reveal potential drug targets and mechanisms to combat infection.
- Studying Immune Evasion: Tests that evaluate the immune response to infection highlight how pathogens evade the immune system, which can guide immunotherapy research.