Introduction
- Cryptococcosis is a serious fungal infection caused by species of the genus Cryptococcus, particularly Cryptococcus neoformans and Cryptococcus gattii.
- These encapsulated yeasts are primarily found in the environment, notably in soil contaminated with pigeon droppings and decaying wood.
- Cryptococcosis predominantly affects immunocompromised individuals, especially those with HIV/AIDS, organ transplant recipients, and individuals receiving immunosuppressive therapy.
- The infection can lead to severe pulmonary, neurological, and systemic disease.
- Cryptococcus species are opportunistic pathogens capable of causing infections when the host’s immune defenses are weakened.
- Diagnosis requires a combination of clinical suspicion, laboratory tests, and identification of the organism in clinical specimens.
Etiology
The etiological agents of cryptococcosis are two main species within the genus Cryptococcus:
- Cryptococcus neoformans: This species is responsible for most cryptococcal infections. It is often isolated from pigeon droppings, decaying wood, and soil. C. neoformans primarily causes pulmonary infections, which can disseminate to the central nervous system (CNS), leading to cryptococcal meningitis.
- Cryptococcus gattii: This species is primarily found in tropical and subtropical regions and has been associated with infections in immunocompetent individuals, unlike C. neoformans, which predominantly affects the immunocompromised. C. gattii is commonly found in soil and trees, particularly eucalyptus trees in certain regions.
Specimens
The types of clinical specimens used to diagnose Cryptococcus infections depend on the site of infection. Common specimens include:
- Cerebrospinal Fluid (CSF): Most commonly obtained in cases of suspected cryptococcal meningitis. It is examined for the presence of Cryptococcus using various diagnostic tests, including India ink staining and culture.
- Blood Cultures: In cases of disseminated cryptococcosis, Cryptococcus can be isolated from blood. Blood cultures are essential in diagnosing systemic infections.
- Sputum or Respiratory Specimens: These are collected from the lower respiratory tract in patients with pulmonary cryptococcosis. It may also include bronchoalveolar lavage (BAL) fluid.
- Tissue Biopsy: For patients with deep or disseminated infections, biopsy samples from affected tissues (e.g., skin lesions or lung tissue) may be required for diagnosis.
- Urine: Urinary tract infections caused by Cryptococcus are uncommon but can be diagnosed by urine culture in some cases.
- Skin Scraps: In cases of cutaneous cryptococcosis, scrapings from skin lesions can be examined.
Direct Microscopic Examination
Direct microscopic examination is one of the primary methods used in diagnosing cryptococcosis. The most commonly used techniques include:
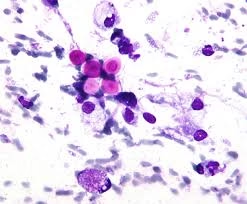
- India Ink Staining: This is one of the most widely used methods for diagnosing Cryptococcus in CSF. The India ink stain highlights the thick, gelatinous capsule surrounding the yeast cells, which appears as a clear halo around the yeast cell. This method is simple and rapid but may have limited sensitivity, particularly in cases with low fungal burden.
- Gram Staining: Cryptococcus appears as spherical or ovoid yeast cells that their thick capsule can identify. The yeast cells may show budding, and they are Gram-positive.
- Mucicarmine Staining: This specialized stain can highlight the polysaccharide capsule of Cryptococcus, which appears red against a yellow background. This method is particularly useful in histopathology for tissue samples.
- KOH Preparation: In some cases, potassium hydroxide (KOH) mounts may be used to prepare clinical specimens, especially skin lesions or respiratory samples, to detect fungal elements. The yeast cells in Cryptococcus are often round and may appear with a clear capsule under the microscope.
- H&E Staining: Hematoxylin and eosin (H&E) staining can be used to examine tissue biopsies. Cryptococcus appears as round yeast cells with a prominent capsule in tissue samples, which can be seen in the tissue sections.
Culture and Identification
Culturing Cryptococcus is essential for definitive diagnosis. The following steps are typically involved:
- Culture Media: Cryptococcus grows well on standard fungal media such as Sabouraud dextrose agar (SDA) and bird seed agar, which is selective for Cryptococcus species. On SDA, colonies are typically creamy, white, or pink and may become mucoid, which reflects the presence of the yeast’s polysaccharide capsule.
- Growth Characteristics: Cryptococcus grows relatively slowly, taking 2–5 days to form colonies. The colonies are generally smooth and glistening and may exhibit a creamy, off-white, or pinkish appearance.
- Biochemical Testing: After initial growth, biochemical tests, including urease production, identify Cryptococcus. Cryptococcus neoformans is urease-positive, and it also produces a positive result on tests such as the fermentation of glucose.
- Antigen Testing: One of the most important methods for identifying Cryptococcus in clinical specimens is the detection of cryptococcal antigen (CrAg). This test can be performed on CSF, serum, urine, or other body fluids. It is highly sensitive and specific, especially for diagnosing cryptococcal meningitis. Lateral flow assays, enzyme immunoassays (EIA), and latex agglutination tests are commonly used for CrAg detection.
- PCR: Polymerase chain reaction (PCR) testing is increasingly used for species identification and detecting Cryptococcus DNA in clinical specimens. PCR is useful for confirming the organism’s presence in cases of negative or slow-growing culture.
Other Laboratory Tests
In addition to direct microscopic examination and culture, several other laboratory tests are important for diagnosing cryptococcosis and assessing its severity:
- Cryptococcal Antigen (CrAg) Test: This is a rapid and highly sensitive test for detecting the polysaccharide capsule of Cryptococcus in clinical specimens. A positive CrAg test in CSF or serum confirms the diagnosis of cryptococcosis. It is especially useful in diagnosing meningitis, as CSF may be difficult to culture.
- Serological Tests: Serological tests for antibodies against Cryptococcus can sometimes be used, though antigen detection is generally preferred for its higher sensitivity.
- Fungal Culture and Susceptibility Testing: For patients with severe or refractory infections, antifungal susceptibility testing may be conducted to guide therapy. Broth dilution or disk diffusion methods can determine antifungal agents’ minimum inhibitory concentration (MIC).
- Imaging: In cases of pulmonary cryptococcosis, chest X-rays or CT scans may reveal nodular lesions or other signs of lung involvement. For suspected cryptococcal meningitis, brain imaging (MRI or CT scan) may show findings such as hydrocephalus or mass effect due to fungal meningitis.
Pathogenesis
The pathogenesis of cryptococcosis is influenced by the yeast’s ability to evade the immune system, invade tissues, and cause disease. The key mechanisms include:
- Capsule Formation: The polysaccharide capsule of Cryptococcus is its primary virulence factor. The capsule helps the yeast evade phagocytosis by immune cells, inhibiting the host’s immune response. It also assists in survival within tissues by reducing the recognition of the pathogen by the immune system.
- Melanin Production: Cryptococcus synthesizes melanin within its cell wall, which may protect it from oxidative stress caused by host immune responses, particularly in the lungs and brain.
- Thermotolerance: Cryptococcus species can grow at body temperature (37°C), a key adaptation that allows them to infect warm-blooded hosts, including humans.
- Invasion of the Central Nervous System: Cryptococcus is known for its ability to cross the blood-brain barrier, leading to cryptococcal meningitis. The yeast can enter the CNS via the bloodstream and evade immune surveillance, often causing chronic inflammation.
- Immune Evasion: In immunocompromised hosts, such as those with HIV/AIDS or organ transplant recipients, the immune system’s inability to mount a sufficient response allows Cryptococcus to establish systemic infections, including meningitis and pulmonary cryptococcosis.
Treatment of Cryptococcosis
Treatment for cryptococcosis depends on the severity of the infection, the species of Cryptococcus, and the patient’s underlying health status. Treatment options include:
- Induction Therapy: The standard induction treatment involves amphotericin B (typically liposomal amphotericin B) combined with flucytosine for severe or disseminated infections, including cryptococcal meningitis. Amphotericin B binds to ergosterol in the fungal cell membrane, causing cell death, while flucytosine inhibits fungal DNA synthesis.
- Consolidation Therapy: Patients are typically switched to fluconazole for consolidation therapy after induction therapy. Fluconazole is an azole antifungal that inhibits the synthesis of ergosterol, a key component of the fungal cell membrane. It is administered for several months to prevent relapse.
- Maintenance Therapy: In immunocompromised patients, prolonged maintenance therapy with fluconazole may be required to prevent the recurrence of the infection.
- Prophylaxis: In patients with HIV/AIDS or other immunocompromised states, prophylactic antifungal therapy (typically with fluconazole) may be prescribed to prevent cryptococcosis.
- Surgical Intervention: In some cases, surgical drainage of cryptococcal lesions, such as in pulmonary cryptococcosis or abscesses, may be necessary.