
Introduction
- The Widal test is a serological diagnostic method widely used to identify typhoid and paratyphoid fevers, collectively known as enteric fever.
- The bacteria Salmonella typhi and Salmonella paratyphi cause these diseases.
- The test detects specific agglutinating antibodies—O (somatic) and H (flagellar)—produced in response to these antigens in the patient’s serum.
- While simple and rapid, the Widal test’s accuracy is influenced by prior vaccination, background antibody levels in endemic areas, and co-existing infections.
Principle
When a known suspension of killed Salmonella antigens (O and H) is mixed with the patient’s serum, the antibodies (if present) in the serum react specifically with their corresponding antigens and produce visible clumping or agglutination.
-
-
O antigen → a somatic antigen (heat-stable, polysaccharide in nature).
→ produces a granular agglutination. -
H antigen → a flagellar antigen (heat-labile, protein in nature).
→ produces a floccular (loose, cotton-wool–like) agglutination.
-
The degree of agglutination (titer) indicates the concentration of antibodies, which helps in diagnosing typhoid or paratyphoid fever.
Requirements
- Samples:
- Serum is the preferred sample. Collect 2–3 mL of venous blood in a sterile container and allow it to clot.
- Centrifuge to separate serum.
- Reagents:
- Commercially prepared Salmonella antigen suspensions, including:
- S. typhi O antigen
- S. typhi H antigen
- S. paratyphi A O and H antigens
- S. paratyphi B O and H antigens
- Physiological saline (if serial dilution is needed).
- Commercially prepared Salmonella antigen suspensions, including:
- Equipment:
- Clean glass slides.
- Applicator sticks or mixing rods.
- Micropipettes and tips for accurate measurement.
Slide Agglutination Method (Qualitative Test)
Procedure
-
Label four separate circles on a clean glass slide as O, H, AH, and BH.
-
Place one drop (approximately 40–50 µL) of undiluted patient serum into each labelled circle.
-
Add one drop of the corresponding Salmonella antigen suspension into each circle:
-
O → S. typhi “O” antigen
-
H → S. typhi “H” antigen
-
AH → S. paratyphi A “H” antigen
-
BH → S. paratyphi B “H” antigen
-
-
Mix the serum and antigen thoroughly in each circle using separate clean applicator sticks.
-
Gently rock the slide manually in a circular motion or place it on a mechanical rotator at about 80–100 rpm.
-
Observe for agglutination within 1 minute; confirm again at 2 minutes.
-
Do not interpret after 2 minutes as drying may cause false-positive results.
-
-
Compare each reaction with positive and negative control sera tested in parallel.
-
Interpretation:
-
Agglutination present → Reactive (positive) for that antigen.
-
No agglutination → Non-reactive (negative).
-
-
Record results. Reactive samples must be confirmed by a quantitative (tube) Widal test to determine antibody titre.
Semi-Quantitative Method (Titration)
- Serially dilute the patient’s serum in saline (1:20, 1:40, 1:80, 1:160, and so on).
- Mix each dilution with an equal volume of antigen suspension.
- Observe for the highest dilution that still shows visible agglutination (titer).
Requirements
- Samples
- Serum:
- Collect 2–3 mL of venous blood aseptically in a plain (clot) tube.
- Allow the blood to clot, and separate the serum by centrifugation at 2,500–3,000 rpm for 10 minutes.
- Use fresh serum or store at 2–8°C for short durations (up to 24 hours).
- Reagents
- Standardized Salmonella Antigen Suspensions (commercially available):
- S. typhi O antigen.
- S. typhi H antigen.
- S. paratyphi A O and H antigens.
- S. paratyphi B O and H antigens.
- S. paratyphi C O and H antigens (less common).
- Physiological saline: For preparing serial dilutions.
- Equipment
- Clean test tubes (12 × 75 mm or equivalent).
- Test tube racks.
- Micropipettes or graduated droppers.
- Water bath or incubator (maintain 37°C).
- Timer or clock.
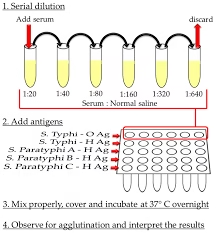
Master Dilution Preparation
Objective: Create serial dilutions to determine antibody concentration.
Steps:
-
Label a series of clean test tubes as 1:20, 1:40, 1:80, 1:160, 1:320, 1:640, and so on.
-
Add 1.9 mL of physiological saline to the first tube (1:20).
-
Add 1.0 mL of saline to all remaining tubes.
-
Pipette 0.1 mL of patient serum into the first tube (1:20) and mix thoroughly.
-
This gives a 1:20 dilution of serum.
-
-
Transfer 1.0 mL from the first tube to the second tube (1:40) and mix well.
-
Continue serially transferring 1.0 mL from each tube to the next (1:80 → 1:160 → 1:320 → 1:640), mixing each time.
-
After mixing the last tube, discard 1.0 mL from it to maintain an equal final volume in all tubes.
-
The series now represents doubling dilutions of the patient’s serum, ready for quantitative Widal agglutination testing.
Procedure
-
Preparing the Test
-
Label the test tubes for each antigen type as follows:
-
Salmonella typhi O
-
Salmonella typhi H
-
Salmonella paratyphi A O/H
-
Salmonella paratyphi B O/H
-
-
Prepare serial (master) serum dilutions in physiological saline as previously described (e.g., 1:20, 1:40, 1:80, 1:160, 1:320, 1:640).
-
Each series of dilutions will correspond to one antigen type.
Adding Antigen
-
To each tube in the dilution series, add 0.5 ml of the respective Salmonella antigen suspension.
-
After adding the antigen, the final volume in each tube should be 1.0 mL.
-
Prepare separate antigen series for each of the four antigens (O, H, AH, BH).
-
Do not interchange pipettes between antigen types to prevent cross-contamination.
Mixing
-
Gently mix the contents of each tube by tapping or by slowly rolling the tube between your palms.
-
Avoid vigorous shaking or bubbles, as these can interfere with agglutination reading.
-
Ensure that all antigen and serum are evenly mixed before incubation.
Incubation
-
Place all tubes upright in a water bath or incubator maintained at 37°C.
-
Incubate for 16–18 hours (usually overnight).
-
After incubation, remove the tubes carefully without disturbing the sediment.
Reading the Results
-
After incubation, gently tilt each tube to observe the pattern of agglutination.
-
Positive reaction: visible agglutination with a clear supernatant above the clumps.
-
Negative reaction: uniformly turbid suspension without visible clumps.
-
Record the highest dilution showing definite agglutination as the antibody titer for that antigen (e.g., TH = 1:160).
Controls
-
Positive Control: Include a tube containing serum with known Salmonella antibodies to verify the reactivity of the antigen.
-
Negative Control: Include a tube containing physiological saline and antigen (no serum) to ensure the antigen does not show auto-agglutination.
-
Results are valid only if:
-
Positive control shows expected agglutination.
-
Negative control remains smooth and uniform.
-
-
Results
Observation
- After incubation, observe the tubes for agglutination.
- Positive results show clumps or granules, while the remaining liquid becomes clearer.
- Negative results appear as uniform turbidity without clumping.
Titer Determination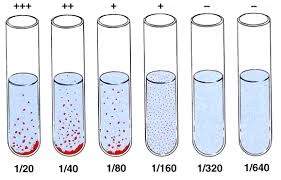
- The titer is the serum’s highest dilution (lowest concentration) that shows visible agglutination.
- Example: If the 1:160 dilution shows agglutination but the 1:320 dilution does not, the antibody titer is 1:160.
Titer Thresholds for Diagnosis
- O antibody: Titers ≥1:160 are significant for active typhoid fever in endemic areas.
- H antibody: High titers (>1:320) may indicate past infection, convalescence, or vaccination.
Paired Sera Testing
- Collect a second serum sample after 7–10 days to observe a fourfold titer rise, confirming active infection.
Clinical Significance
1. Diagnostic Applications
-
Acute Typhoid Fever:
A high titer of S. typhi O antibodies (≥1:160) indicates an active or recent infection, as O antibodies appear early and rise rapidly during the acute phase. -
Chronic Carrier State:
Persistently elevated H antibody titers in the absence of significant O titers suggest a carrier state, where the individual harbors the bacteria without active illness. -
Paratyphoid Fever:
Agglutination with S. paratyphi A or B antigens confirms infection by these related strains, aiding differentiation from S. typhi infection.
2. Monitoring Response to Treatment
-
Falling antibody titers during or after therapy indicate clinical improvement and effective treatment response.
-
Persistently high or rising titers may reflect ongoing infection, relapse, or reinfection.
3. Epidemiological Importance
-
-
The Widal test is valuable in epidemiological surveys to assess the prevalence of enteric fever and population immunity in endemic regions.
-
It helps in identifying outbreaks, carrier reservoirs, and guiding public health control measures.
-
Limitations
- Cross-Reactivity: Antibodies against other bacteria (e.g., E. coli, Proteus) may cause false positives.
- Vaccination History: Recent vaccination against typhoid may yield elevated titers.
- Background Antibodies: In endemic areas, baseline titers may already be high due to prior exposure.
- Carrier State: Low-level titers may persist in asymptomatic carriers.
Precautions
- Use fresh reagents to ensure reliable results.
- Avoid contamination of antigen suspensions.
- Ensure proper incubation at 37°C for accurate agglutination.
- Use positive and negative controls to validate the test setup.
