Introduction
- The Rose-Waaler Brucella Agglutination Test is a serological test detecting Brucella-specific antibodies in human serum.
- Brucella species are intracellular bacteria causing brucellosis, a zoonotic disease characterized by fever, malaise, and systemic inflammation.
- The disease is contracted through contact with infected animals, ingesting unpasteurized dairy, or inhaling aerosols.
- This test is particularly useful as a screening method for diagnosing brucellosis.
- The immune system of an infected individual produces antibodies against Brucella antigens, which can be detected through agglutination reactions.
- The test uses inactivated Brucella antigens to detect specific antibodies (IgM and IgG) in the patient’s serum.
Principle
The Rose-Waaler test works on the agglutination principle, where antigens from Brucella bacteria react with specific antibodies in the patient’s serum to form visible clumps (agglutinates).
Key Details of the Reaction:
- Antigen: Brucella antigens are prepared from killed Brucella strains.
- Antibody: The patient’s serum may contain antibodies (mainly IgM and IgG) produced in response to infection.
- Visual Endpoint: Agglutination occurs when antigen-antibody complexes form a visible network.
- Qualitative Test: Determines the presence of antibodies (positive or negative result).
- Quantitative Test: Estimates antibody concentration through serial serum dilutions.
Requirements
Sample
- Type: Serum (preferred). Plasma (EDTA or heparinized) may also be used.
- Volume: 2–3 mL of venous blood is sufficient.
- Collection and Storage:
- Use sterile equipment to collect blood and centrifuge promptly to obtain serum.
- Store serum at 2–8°C if testing within 72 hours; for extended storage, freeze at −20°C. Avoid multiple freeze-thaw cycles.
Reagents and Materials
- Brucella Antigen:
- Prepared from killed Brucella abortus, B. melitensis, or B. suis. The antigens may be stained with dyes like Rose Bengal for better visibility.
- Diluent:
- Normal saline or phosphate-buffered saline (PBS) for preparing serial dilutions.
- Positive Control Serum:
- A serum sample with a known Brucella antibody titer was used to validate the test.
- Negative Control Serum:
- CRP-free serum or saline to ensure test specificity.
- Glassware and Equipment:
- Clean glass slides (for qualitative testing).
- Tubes for serial dilutions in quantitative testing.
- Pipettes (automatic or manual) for accurate reagent handling.
- Tube racks and a calibrated rotator (if required).
- Other Items:
- Micropipettes, mixing sticks, and disposable tips.
- Timer to monitor reaction time.
Procedure
Slide Agglutination Test (Qualitative Method)
This is a rapid screening method to detect the presence of Brucella antibodies.
Steps:
- Label a clean glass slide for identification.
- Place one drop (50 µL) of patient serum on the slide.
- Add an equal volume of Brucella antigen (50 µL) to the serum.
- Mix the antigen and serum or gently rotate the slide using a stick.
- Observe for agglutination (visible clumping) within 2–5 minutes.
Interpretation: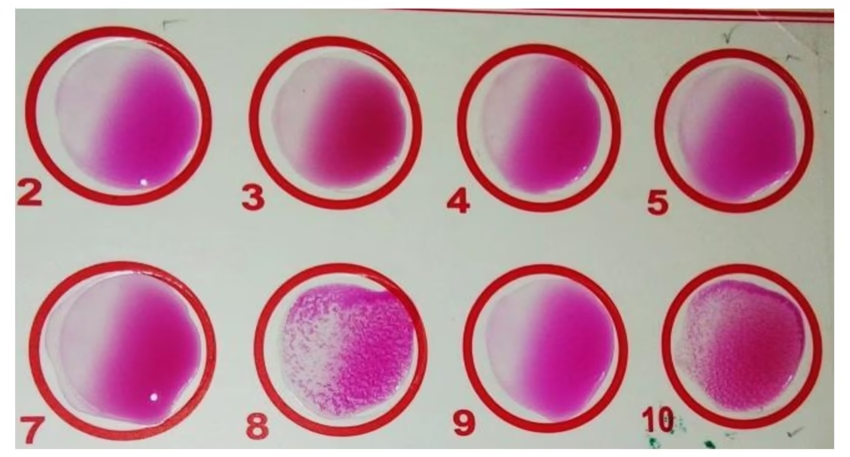
- Positive: Visible clumping indicates the presence of antibodies against Brucella.
- Negative: No clumping indicates the absence of detectable antibodies.
Tube Agglutination Test (Quantitative Method)
This method determines the antibody titer in the patient’s serum through serial dilutions.
Steps:
- Prepare serial dilutions of the patient’s serum in saline or PBS (e.g., 1:10, 1:20, 1:40, 1:80, etc.).
- Add 0.5 mL of each diluted serum into labeled test tubes.
- Add 0.5 mL of Brucella antigen suspension to each tube.
- Mix thoroughly and incubate the tubes at 37°C for 18–24 hours.
- After incubation, observe the tubes for agglutination.
Interpretation:
- The highest serum dilution showing at least 50% agglutination is recorded as the antibody titer.
Results
Qualitative Results:
- Positive: Presence of agglutination (clumping). Indicates exposure to Brucella.
- Negative: Absence of agglutination. No detectable antibodies.
Quantitative Results:
- Titer: Expressed as the reciprocal of the highest serum dilution, showing significant agglutination.
Diagnostic Criteria:
- <1:40: Negative or insignificant titer, unlikely brucellosis.
- 1:80 to 1:160: Suggestive of exposure or early infection.
- >1:160: Indicates active infection or significant exposure.
Clinical Significance
- Diagnosis of Brucellosis:
- A positive result strongly supports the diagnosis, especially when correlated with clinical signs like fever, sweating, joint pain, and hepatosplenomegaly.
- Monitoring Disease Progression:
- Rising titers in sequential tests confirm an ongoing or worsening infection.
- Decreasing titers indicates recovery or successful treatment.
- Differential Diagnosis:
- Helps differentiate brucellosis from other febrile illnesses.
- Epidemiological Surveillance:
- Useful in regions where brucellosis is endemic, especially among occupationally exposed groups (e.g., farmers, and veterinarians).
Limitations
- Non-Specificity:
- Cross-reactivity with other Gram-negative bacteria, such as Yersinia enterocolitica, Escherichia coli, or Francisella tularensis, may produce false positives.
- Timing of Testing:
- Antibodies may not be detectable during the early stages of infection. A repeat test after 1–2 weeks is recommended for better sensitivity.
- Chronic Brucellosis:
- Persistently high antibody titers may indicate chronic infection but do not always indicate active disease.
- Test Performance:
- The sensitivity and specificity depend on the quality of antigens and reagents used.
Precautions
- Sample Collection:
- Avoid hemolysis or contamination during blood collection.
- Reagent Quality:
- Ensure standardized Brucella antigens and proper storage conditions.
- Correlate with Clinical Data:
- Positive results must always be interpreted alongside clinical findings and other diagnostic tests, such as PCR or blood culture.
- Repeat Testing:
- Repeating testing with serial samples may provide clarity if initial results are inconclusive.