Principle
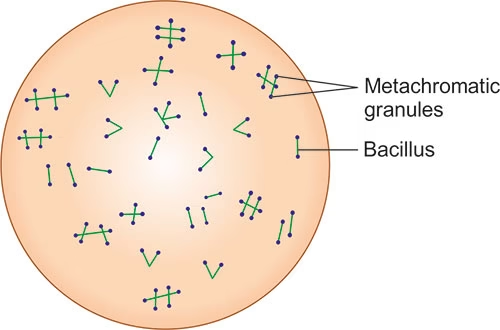
- Albert’s stain works based on the unique ability of Corynebacterium species to accumulate metachromatic granules in their cytoplasm.
- These granules are a result of the accumulation of polyphosphate.
- The staining method relies on the use of a special stain that binds to these granules, turning them blue-black, while the rest of the cell takes on a greenish hue.
- The principle of Albert’s staining involves the use of a combination of dyes that selectively stain the bacterial cells and their granules.
- The metachromatic granules of Corynebacterium species will appear as dark blue-black inclusions under the microscope, making them easily distinguishable from the rest of the cell.
Requirements
To perform Albert’s staining, you will need the following materials:
-
Microscope: A compound light microscope, preferably with an oil immersion lens (100x) for high magnification.
-
Glass slides: For preparing bacterial smears.
-
Inoculating loop: To transfer the bacterial sample onto the slide.
-
Heat source: To fix the smear onto the slide.
-
Staining rack: To hold the slides during staining.
-
Distilled water: For rinsing the slides.
-
Staining containers: To hold the staining reagents.
Reagents
-
Albert’s Stain Solution: This is a mixture of two primary components:
-
Alberts’ Solution A: Contains a combination of methyl violet and iodine, which stains the bacteria and binds to the granules.
-
Alberts’ Solution B: Contains potassium iodide, which aids in the iodine staining and helps to intensify the color of the granules.
-
-
Acid-alcohol solution: This is used as a decolorizing agent to remove excess stain from non-granular areas.
Sample
-
Bacterial Culture: Albert’s stain is most commonly used to detect Corynebacterium diphtheriae in clinical specimens. The sample can be collected from various sources such as:
-
Throat swabs: For suspected diphtheria cases.
-
Tissue biopsies: For diagnosing infections caused by Corynebacterium species.
-
Sputum and other respiratory samples: For patients with respiratory symptoms suggestive of diphtheria.
-
-
Smear Preparation: A small sample of the bacteria is spread onto a glass slide to create a thin smear. This is then air-dried and heat-fixed to ensure that the bacteria stay attached to the slide during the staining process.
Procedure
-
Prepare the smear:
-
Place a small amount of the bacterial sample on a clean glass slide.
-
Using an inoculating loop, spread the sample into a thin, even layer.
-
Allow the smear to air dry completely.
-
Heat-fix the smear by gently passing the slide through a flame 2-3 times to ensure that the bacteria stick to the slide.
-
-
Stain with Albert’s Solution A:
-
Apply a few drops of Albert’s Solution A (the methyl violet and iodine mixture) onto the smear. Ensure the entire smear is covered with the stain.
-
Allow the stain to sit for 5-10 minutes at room temperature.
-
-
Decolorize with acid-alcohol:
-
Gently rinse the slide with an acid-alcohol solution to remove excess stain from the background. Be careful not to wash away the granules.
-
Rinse with distilled water to stop the decolorization process.
-
-
Apply Albert’s Solution B:
-
Apply Albert’s Solution B (containing potassium iodide) onto the smear and leave it for an additional 5 minutes.
-
This step intensifies the staining of the granules and helps in further enhancing their visibility.
-
-
Final rinse and drying:
-
Rinse the slide with distilled water to remove any excess stain.
-
Allow the slide to air dry completely.
-
-
Microscopic examination:
-
Examine the stained slide under a compound light microscope using an oil immersion lens (100x).
-
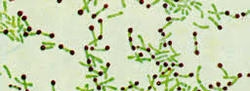
The metachromatic granules will appear as dark blue-black granules within the bacterial cells, while the rest of the bacterial cell will appear greenish.
-
Results
-
Positive Results:
-
The bacteria will show characteristic metachromatic granules that stain dark blue-black.
-
The rest of the bacterial cell will appear greenish.
-
Corynebacterium diphtheriae and other Corynebacterium species will have these darkly stained inclusions, making them easily identifiable.
-
-
Negative Results:
-
If the bacteria do not contain metachromatic granules, they will not show the characteristic blue-black staining.
-
The cells will appear as greenish, with no dark inclusions.
-
Applications
-
Diagnosis of Diphtheria: Albert’s staining is primarily used for identifying Corynebacterium diphtheriae, the bacterium responsible for diphtheria. The presence of metachromatic granules is a key diagnostic feature of this pathogen.
-
Identification of Corynebacterium Species: Albert’s staining helps to identify various Corynebacterium species, especially those that cause opportunistic infections in immunocompromised individuals.
-
Bacterial Research: This method is used in microbiological research to study Corynebacterium species, their morphology, and granule formation.
-
Educational Purpose: Albert’s stain is also used in educational settings to demonstrate the presence of metachromatic granules in bacteria, which serves as an excellent example of a bacterial feature under the microscope.
-
Confirmation of Infection in Clinical Specimens: It is an effective method for confirming the presence of diphtheria-causing bacteria in throat swabs and other clinical samples.