Introduction
- Immunoassays are analytical techniques that use the specific binding between an antigen and an antibody to detect and quantify molecules of biological or clinical importance.
- Since the 1960s, when radioimmunoassay (RIA) was first developed, immunoassay technology has advanced tremendously, introducing alternatives that avoid the drawbacks of radioactive methods.
- Among these, the fluorescent immunoassay (FIA) has emerged as one of the most powerful tools, combining immunological specificity with the high sensitivity of fluorescence detection.

- FIA uses fluorophores as labels instead of radioisotopes or enzymes.
- These fluorophores absorb light at a certain wavelength and emit light at a longer wavelength, allowing sensitive measurement of antigen–antibody interactions.
- Due to its unique properties, FIA has become indispensable in clinical diagnostics, biomedical research, pharmacology, food safety, and environmental monitoring.
Definition
A fluorescent immunoassay (FIA) is a laboratory method used to detect and quantify specific biological molecules (antigens or antibodies) by employing fluorophore-labeled immunoreagents.
-
When a fluorophore-labeled antibody binds to its antigen, or vice versa, the complex can be excited with light of a particular wavelength.
-
The emitted fluorescence is detected, and its intensity is proportional to the concentration of the target molecule.
Thus, FIA is defined as:
“An immunoassay that utilizes fluorescent labels to detect and measure antigen–antibody binding reactions with high sensitivity and specificity.”
Principle
The principle of FIA rests on three key foundations: immunological specificity, fluorescence, and quantitative signal measurement.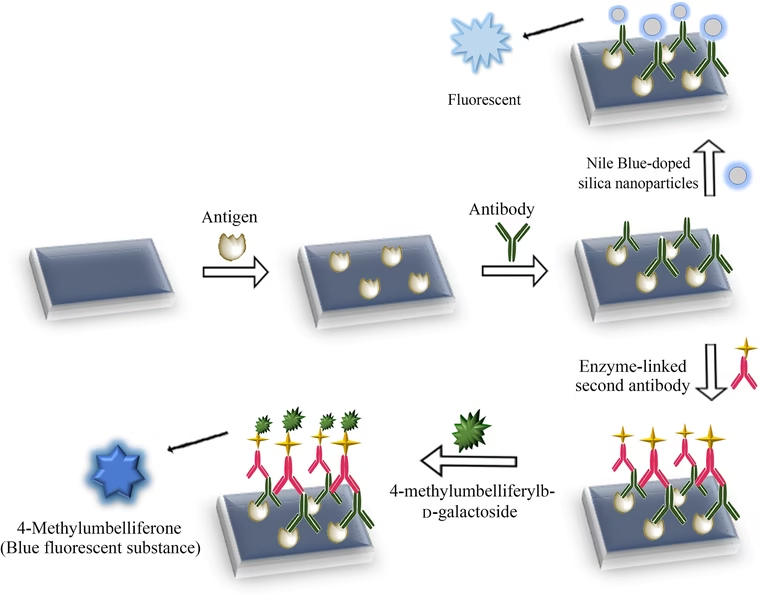
1 Immunological Specificity
-
Antigens are molecules capable of stimulating an immune response.
-
Antibodies are Y-shaped proteins that bind to antigens with high specificity at their antigen-binding sites.
-
This lock-and-key mechanism ensures that FIA provides highly selective results.
2 Fluorescence
-
Fluorescence is the ability of a molecule (fluorophore) to absorb photons at an excitation wavelength and emit photons at a longer emission wavelength.
-
Example: Fluorescein absorbs blue light (~490 nm) and emits green light (~520 nm).
-
By tagging antibodies or antigens with fluorophores, binding events can be visualized or quantified.
3 Quantitative Detection
-
The intensity of emitted fluorescence is proportional to the concentration of antigen–antibody complexes.
-
Specialised instruments such as fluorescence microscopes, plate readers, flow cytometers, and spectrofluorometers are used for measurement.
Components
-
Antigen – The target analyte (e.g., protein, hormone, pathogen, toxin).
-
Antibody – Specific immunoglobulin designed to bind antigen.
-
Fluorophore – The fluorescent label attached to antibody or antigen. Common fluorophores include:
-
Fluorescein isothiocyanate (FITC)
-
Rhodamine
-
Alexa Fluor dyes
-
Cyanine dyes (Cy3, Cy5)
-
Quantum dots (nanoparticle fluorophores)
-
-
Detection System – Device to measure fluorescence (e.g., fluorometer, microscope, microplate reader).
-
Solid Support (optional) – Nitrocellulose, polystyrene beads, or microplates to immobilize immunoreagents.
Types
FIA can be classified based on assay design and detection strategy:
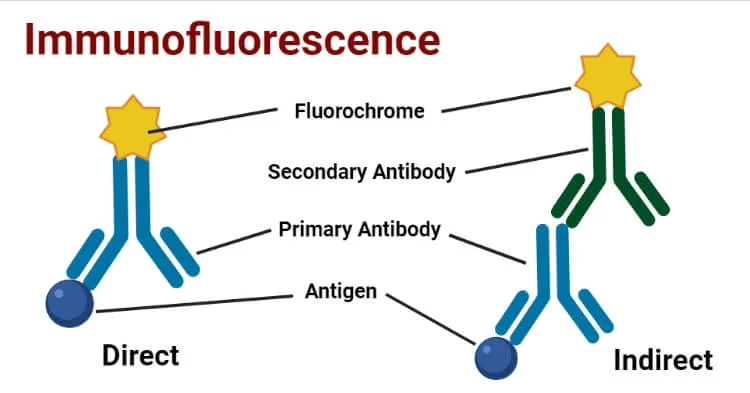
1 Direct Fluorescent Immunoassay (DFA)
-
Antibody specific to the antigen is directly labeled with a fluorophore.
-
When antigen is present, the fluorescent antibody binds and emits signal.
-
Advantages: Fast, fewer steps.
-
Limitations: Lower sensitivity because no signal amplification.
-
Example: DFA test for Rabies virus in brain tissue.
2 Indirect Fluorescent Immunoassay (IFA)
-
Antigen is first bound by an unlabeled primary antibody.
-
A fluorophore-labeled secondary antibody binds the primary antibody.
-
Advantages: Increased sensitivity (multiple secondary antibodies can bind each primary).
-
Example: Detection of antinuclear antibodies (ANA) in autoimmune disease diagnosis.
3 Competitive Fluorescent Immunoassay
-
Labeled antigen and unlabeled antigen (sample) compete for antibody binding sites.
-
Higher concentration of unlabeled antigen → lower fluorescence intensity.
-
Useful for small molecules like drugs, hormones, and toxins.
-
Example: Measurement of cortisol levels in serum.
4 Sandwich Fluorescent Immunoassay
-
Antigen is captured by an immobilized antibody.
-
A second, fluorophore-labeled antibody binds a different epitope.
-
Advantages: Very high sensitivity and specificity.
-
Example: Detection of viral proteins, cytokines, and biomarkers.
5 Fluorescence Polarization Immunoassay (FPIA)
-
Based on differences in rotational motion of molecules.
-
Free fluorescent antigen rotates rapidly → depolarized emission.
-
Antibody-bound antigen rotates slowly → polarized emission.
-
Application: Therapeutic drug monitoring (e.g., theophylline, cyclosporine).
6 Time-Resolved Fluorescent Immunoassay (TR-FIA)
-
Uses special fluorophores (lanthanides like Europium, Terbium) with long-lived fluorescence.
-
Allows measurement after background autofluorescence has decayed.
-
Advantages: Very high sensitivity, reduced noise.
-
Applications: Detection of very low-abundance biomarkers in clinical samples.
7 Multiplex Fluorescent Immunoassay
-
Uses beads, arrays, or microchips coated with different antibodies, each tagged with distinct fluorophores.
-
Detects multiple analytes simultaneously in a single sample.
-
Example: Luminex xMAP technology – simultaneous detection of 50+ cytokines.
8 Flow Cytometry-Based FIA
-
Fluorescently labeled antibodies bind to cell-surface or intracellular markers.
-
Laser excitation detects fluorescence from individual cells.
-
Application: Immunophenotyping of blood cells, cancer diagnostics, HIV monitoring (CD4 counts).
Applications
FIA has wide-ranging applications across many fields:
1 Clinical Diagnostics
-
Infectious Diseases:
-
DFA for Rabies virus, Chlamydia trachomatis, Respiratory syncytial virus.
-
-
Endocrinology:
-
Measurement of insulin, thyroid hormones, cortisol.
-
-
Oncology:
-
Tumor markers (PSA, CA-125, CEA).
-
-
Autoimmune Disorders:
-
Indirect immunofluorescence for ANA in lupus and other autoimmune diseases.
-
-
Hematology:
-
Blood group antigen typing.
-
2 Biomedical Research
-
Localization of proteins in cells using immunofluorescence microscopy.
-
Tracking cellular signaling pathways.
-
Analysis of protein–protein interactions.
-
High-throughput screening of drug candidates.
3 Pharmaceutical Industry
-
Therapeutic drug monitoring (FPIA assays for antibiotics, immunosuppressants).
-
Pharmacokinetics and pharmacodynamics studies.
-
Biomarker discovery and validation in clinical trials.
4 Food and Agriculture
-
Detection of foodborne pathogens (Salmonella, E. coli O157:H7).
-
Identification of allergens (gluten, peanut proteins).
-
Monitoring of pesticide residues.
5 Environmental Monitoring
-
Detection of toxins in water (cyanobacterial microcystins).
-
Monitoring microbial contamination in soil and water.
-
Identification of endocrine-disrupting chemicals.
6 Point-of-Care Testing
-
Portable FIA devices for bedside testing.
-
Examples:
-
COVID-19 antigen fluorescent assays.
-
Troponin tests for myocardial infarction.
-
Rapid flu and dengue antigen detection kits.
-
Advantages
-
High Sensitivity – Detects femtomolar–picomolar concentrations.
-
High Specificity – Due to antigen–antibody recognition.
-
Multiplexing Capability – Multiple analytes can be measured simultaneously.
-
Wide Dynamic Range – Works over broad concentration ranges.
-
Non-radioactive – Safer than radioimmunoassays.
-
Versatility – Can be adapted to microscopy, flow cytometry, microarrays, or plate assays.
-
Quantitative and Qualitative – Provides both presence/absence and precise concentration.
Limitations
-
Autofluorescence – Some biological samples (blood, tissues) emit natural fluorescence that interferes with signal.
-
Photobleaching – Fluorophores lose fluorescence when exposed to light for prolonged periods.
-
Quenching – Fluorescence can be reduced by chemical interactions or environmental conditions.
-
Cost – High-quality fluorophores and instruments are expensive.
-
Technical Expertise – Requires trained personnel for accurate execution.
-
Cross-Reactivity – Non-specific binding can give false positives.
-
Hook Effect – Extremely high antigen concentration may give falsely low results.
-
Stability Issues – Some fluorophores degrade under temperature or pH changes.
Recent Advances in FIA
-
Quantum Dots (QDs): Nanoparticles with exceptional brightness and photostability.
-
Near-Infrared (NIR) Fluorophores: Reduce background noise and allow deeper tissue penetration.
-
Microfluidic FIA Systems: Lab-on-a-chip devices for rapid, portable analysis.
-
Smartphone-Based FIA: Mobile phone cameras used for fluorescence detection in low-resource settings.
-
AI and Image Analysis: Automated interpretation of immunofluorescence microscopy.
-
Dual-Label and Ratiometric FIAs: Improve accuracy by reducing background fluctuations.