
Introduction
Western blotting, also called immunoblotting, is a laboratory technique used to detect, identify, and semi-quantify specific proteins from a complex mixture of biological samples such as cell lysates, tissue homogenates, serum, or other body fluids.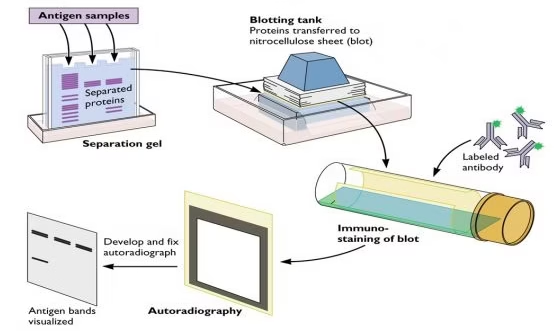
-
It combines SDS-PAGE (for separation of proteins by size) with antibody-based immunodetection (for specificity).
-
The term “Western blot” originated in analogy with the Southern blot (DNA detection method) and Northern blot (RNA detection method). Since this method detects proteins, it was humorously named the “Western blot.”
-
First described by Towbin et al. in 1979, it has since become a gold standard tool in research and clinical diagnostics.
Western blotting remains a cornerstone of molecular biology, immunology, virology, neuroscience, and medical research, especially because it allows confirmation of protein presence and provides evidence for gene expression at the protein level.
Principle
The principle of Western blotting is based on: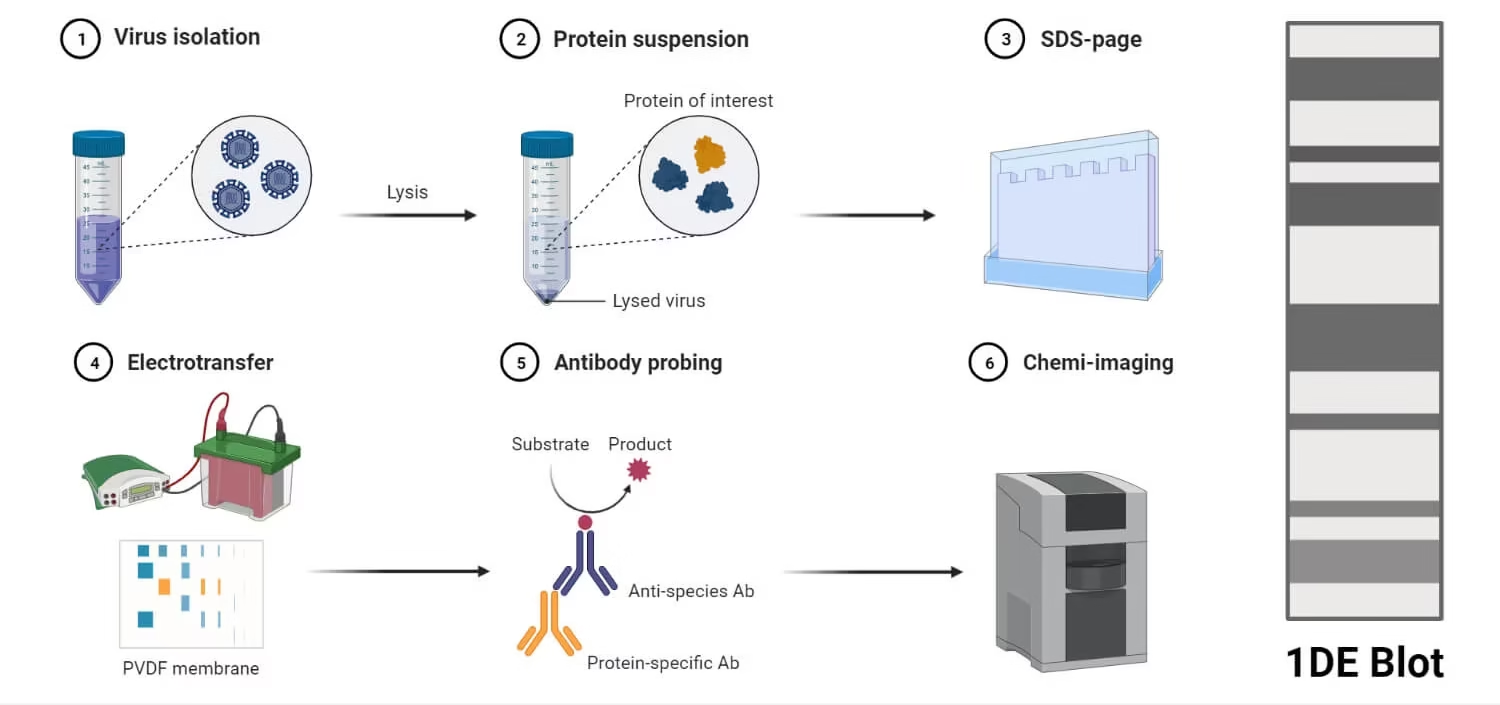
-
Protein Separation by Size
-
Proteins are denatured with detergent (SDS) and separated on a polyacrylamide gel by SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis).
-
Smaller proteins migrate faster through the gel pores, larger proteins move slower.
-
-
Transfer to Membrane
-
Separated proteins are transferred from the gel onto a nitrocellulose or PVDF membrane, which binds proteins.
-
This step makes proteins accessible to antibodies for detection.
-
-
Antigen–Antibody Interaction
-
The membrane is incubated with a primary antibody specific to the target protein.
-
A secondary antibody, conjugated to an enzyme (e.g., horseradish peroxidase) or fluorophore, binds the primary antibody.
-
Upon addition of a substrate, a detectable signal (chemiluminescence, fluorescence, or color) is produced.
-
-
Detection and Analysis
-
The signal corresponds to the location of the protein on the membrane.
-
Band intensity is proportional to protein quantity.
-
In short:
Western blot works by separating proteins by size, transferring them to a membrane, and detecting specific proteins with antibody-based labeling.
Steps of Western Blotting
Western blotting involves several key steps:
Step 1: Sample Preparation
-
Cells or tissues are lysed to extract proteins.
-
Protease inhibitors are added to prevent degradation.
-
Protein concentration is measured (e.g., Bradford or BCA assay).
Step 2: Gel Electrophoresis (SDS-PAGE)
-
Proteins are mixed with SDS (denatures proteins, coats them with negative charge).
-
Heat is applied to fully denature proteins.
-
Samples are loaded into polyacrylamide gel wells.
-
Electric current is applied: proteins migrate toward the positive electrode.
-
Separation occurs mainly on the basis of molecular weight.
Step 3: Transfer to Membrane
-
Proteins are transferred from gel to a nitrocellulose or PVDF membrane by:
-
Electroblotting (most common): Electric current moves proteins onto membrane.
-
Capillary or vacuum transfer (less common).
-
-
Membrane is now a “replica” of protein separation from the gel.
Step 4: Blocking
-
Membrane has nonspecific binding sites.
-
To prevent antibodies from binding randomly, the membrane is incubated with blocking solution (e.g., BSA, non-fat dry milk).
-
This step ensures specific antibody–antigen binding.
Step 5: Primary Antibody Incubation
-
The membrane is incubated with a primary antibody that specifically binds to the target protein.
-
Antibody–antigen binding provides specificity to the assay.
Step 6: Secondary Antibody Incubation
-
A secondary antibody, which recognizes the primary antibody, is added.
-
This antibody is conjugated with a detection molecule:
-
Enzyme (HRP, AP): Produces chemiluminescence or color when substrate is added.
-
Fluorophore: Allows direct visualization with fluorescence imaging.
-
-
This step amplifies the signal, making detection more sensitive.
Step 7: Detection
-
Depending on the label, detection is done using:
-
Chemiluminescence (most common): Light emitted upon enzyme–substrate reaction is captured on X-ray film or CCD camera.
-
Fluorescence: Detected with imaging systems.
-
Colorimetric detection: Produces visible colored bands.
-
Step 8: Analysis
-
Bands on the membrane indicate presence of specific proteins.
-
Band size (migration distance) indicates molecular weight of the protein.
-
Band intensity correlates with protein quantity.
-
Results are compared with molecular weight markers and controls.
Results Interpretation
Western blot results appear as bands on a membrane:
-
Band Position: Corresponds to molecular weight.
-
Example: If a protein is ~50 kDa, its band will appear at that region of the gel.
-
-
Band Intensity: Reflects protein abundance. Stronger bands = higher concentration.
-
Specificity: Presence of bands at expected molecular weight confirms target protein.
-
Controls:
-
Positive control: Ensures antibody works.
-
Negative control: Ensures specificity (no non-specific binding).
-
Loading control (e.g., actin, GAPDH): Ensures equal protein loading in each lane.
-
Applications
Western blotting is one of the most widely used techniques in life sciences.
1 Clinical Applications
-
HIV Diagnosis: Confirmation test for HIV antibodies.
-
Lyme Disease: Detection of Borrelia burgdorferi antibodies.
-
Prion Diseases: Detection of abnormal prion proteins.
-
Viral Infections: Used in hepatitis, herpes, and other viral research.
2 Research Applications
-
Protein Identification: Detect specific proteins in complex mixtures.
-
Protein Quantification: Estimate protein expression levels.
-
Post-translational Modifications: Detect phosphorylated, glycosylated proteins.
-
Gene Expression Studies: Verify translation of specific genes into proteins.
3 Pharmaceutical Applications
-
Drug Development: Study effects of drugs on protein expression.
-
Biomarker Validation: Identify disease-related proteins.
-
Quality Control: Confirm presence of therapeutic proteins.
4 Forensic and Veterinary Applications
-
Detection of animal diseases (BSE/mad cow disease, scrapie).
-
Protein analysis in forensic investigations.
Advantages
-
High specificity (due to antibody recognition).
-
High sensitivity with enhanced detection methods.
-
Provides both qualitative and semi-quantitative information.
-
Can detect post-translational modifications.
-
Widely validated and reliable.
Limitations
-
Labor-intensive and time-consuming.
-
Requires technical expertise.
-
Antibody quality determines accuracy.
-
Not fully quantitative (better for relative comparisons).
-
It can produce false positives/negatives if controls are poor.