Introduction
- Recombinant DNA (rDNA) refers to DNA molecules created by joining DNA from two or more sources.
- The process typically involves inserting the recombinant DNA into a host organism so that it can replicate or express the genes.
- Recombinant DNA technology enables scientists to isolate, study, modify and combine genes.
- It uses tools like restriction enzymes, DNA ligase, vectors, host cells, and selection markers to manipulate DNA.
- Key benefits include production of medically important proteins (e.g. insulin), gene therapy, modifying crops, and research into gene function.
- It is a foundational technology in biotechnology, agriculture, medicine and basic genetic research.
Tools Used
Here are the major tools:
| Tool | Function / Role | Examples / Types |
|---|---|---|
| Restriction Enzymes (Endonucleases) | Cut DNA at specific recognition sequences into fragments with either blunt or sticky (overhanging) ends. | EcoRI, HindIII, BamHI etc. |
| DNA Ligase | Joins DNA fragments (inserts and vector) by forming phosphodiester bonds, sealing nicks. | |
| Polymerase (DNA polymerase, Reverse transcriptase) | Amplify DNA (PCR), or convert RNA to cDNA if needed. | |
| Vectors (Cloning Vectors) | Carry foreign DNA into host, replicate there; provide features to allow selection and expression. | |
| Host organisms / Cells | Cells that accept the recombinant molecules to replicate and (if needed) express the gene. Usually bacteria (E. coli), yeast, mammalian cells, plant cells. | |
| Selectable Markers | Allow identification of transformed hosts vs non-transformed ones. Usually antibiotic resistance, or metabolic markers. | |
| Multiple Cloning Site (MCS) or Cloning Sites | Places in the vector that have many restriction enzyme cutting sites to facilitate insertion of foreign DNA. | |
| Other Tools & Methods | Gel electrophoresis (to check size, purify fragments), competent cell prep, transformation/transfection techniques (electroporation, chemical, etc.), modern assembly methods (e.g. Gibson, Golden Gate) etc. |
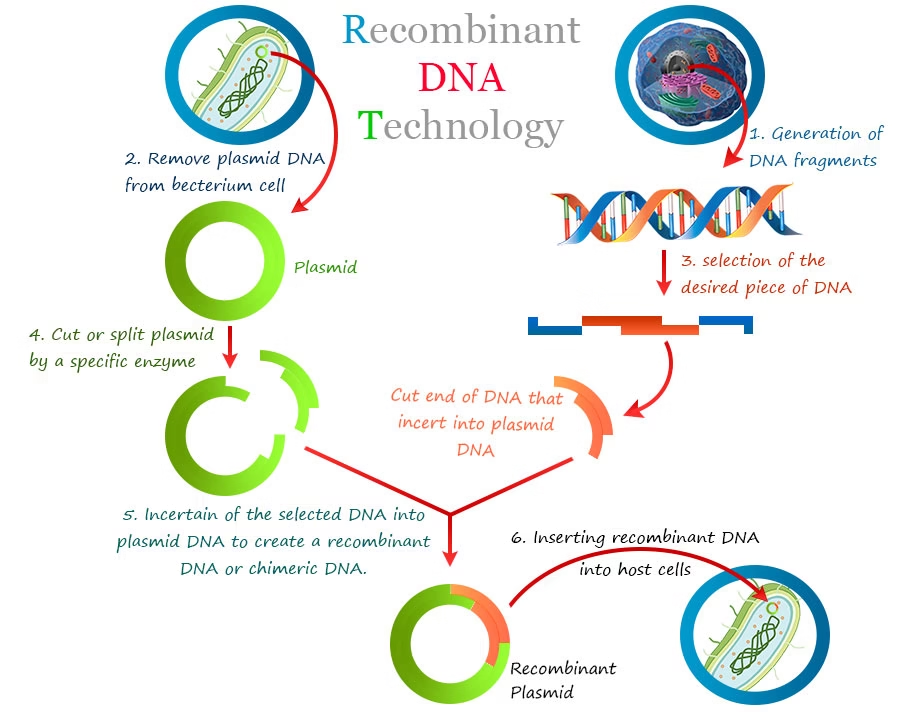
Process of Recombinant DNA Technology
Below is a step-by-step typical workflow, with sub-steps / variants.
-
Isolation of the DNA / gene of interest
-
Source: could be genomic DNA, cDNA (for coding regions), RNA → cDNA.
-
Use of polymerase chain reaction (PCR) if sequence is known; or extraction followed by digestion.
-
-
Digestion with Restriction Enzymes
-
Cut both the vector and the insert DNA using same or compatible restriction enzymes to generate compatible ends (sticky or blunt) for ligation.
-
-
Purification of DNA Fragments
-
After digestion, gel electrophoresis to separate fragments; extraction of the correct band; clean-up to remove contaminating enzymes etc.
-
-
Ligation of Insert into Vector
-
DNA ligase enzyme used to ligate/attach the gene of interest into the vector backbone.
-
-
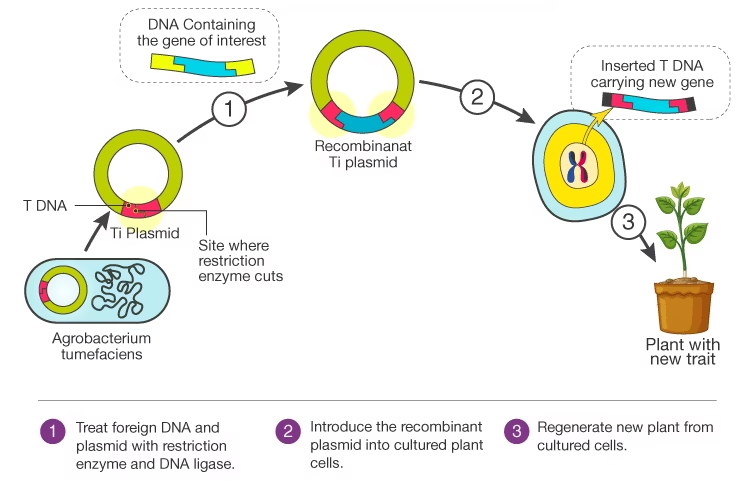
Transformation (or Transfection) of Host Cell
-
Introduce recombinant vector into host cells. Methods: chemical transformation (e.g. CaCl₂ and heat shock), electroporation, micro-injection, Agrobacterium-mediated for plants etc.
-
-
Selection / Screening of Recombinant Cells
-
Using selectable marker genes (e.g. antibiotic resistance) so only transformed cells survive.
-
Additional screening may include blue/white screening (insertion in lacZ etc.), colony PCR, restriction digest check and sequencing.
-
-
Expression (if needed), Propagation & Maintenance
-
Once recombinant cells are identified, allow them to grow to amplify plasmid or expression of the protein product.
-
For expression: promoter in vector, regulatory sequences, maybe inducible promoters etc.
-
-
Analysis / Verification
-
Confirm correct insert, orientation, integrity via sequencing.
-
Assess expression (mRNA level, protein level), functional assays etc.
-
Variants / modern improvements:
-
Assembly methods like Gibson Assembly (joining multiple fragments in one reaction)
-
Golden Gate cloning or Modular Cloning (using Type IIS enzymes) for scarless, modular, multi-part assembly.
Applications
Some of the broad uses:
-
Production of medically important proteins (insulin, growth hormones, therapeutic antibodies)
-
Vaccine development (e.g. recombinant subunit vaccines)
-
Gene therapy: introducing functional gene into patient’s cells to correct defective ones
-
Agricultural biotechnology: transgenic crops with pest resistance, improved yield, nutritional quality etc.
-
Industrial biotechnology: enzymes, biofuels, bioplastics etc.
-
Research: studying gene function, regulatory sequences, protein structure & function etc.
DNA Cloning
Since this is a part of recombinant DNA, here’s what DNA cloning specifically is, and its features:
-
DNA cloning is the process of making multiple, identical copies of a piece of DNA (gene, fragment). Usually involves inserting the DNA fragment into a vector and propagating it in a host organism.
-
Types of Cloning:
-
Gene Cloning / Molecular Cloning: Cloning a particular gene to study it or produce the protein.
-
Sub-cloning: Moving a gene fragment / insert from one vector to another.
-
cDNA Cloning: Cloning DNA made from mRNA; thus representing expressed genes only.
-
Genomic Library Cloning: Cloning many fragments of genome to cover whole genome; used for mapping, sequencing etc.
-
-
Features required in cloning vectors: origin of replication, selectable markers, cloning sites (MCS), promoter if expression desired, reporter genes perhaps.
-
Host considerations: growth rate, ease of transformation, expression capability, post-translational modifications etc.
Applications of Gene Cloning
Here are specific examples / more detailed use cases:
-
Protein production: e.g. producing human insulin in bacteria; recombinant vaccines etc.
-
Functional studies: Mutagenesis, studying regulatory regions; overexpression or knock-out/knock-down.
-
Diagnostic tools: Production of probes, recombinant antigens, gene-based markers etc.
-
Agriculture: Cloning genes for pest resistance (Bt toxin genes), herbicide resistance, drought tolerance, improved nutrition (Golden Rice etc.).
-
Gene Therapy: Cloning therapeutic genes into viral vectors or other delivery systems to correct genetic disorders.
-
Biopharmaceuticals: Antibodies, hormones, enzymes etc.
-
Environmental biotechnology: Degradation pathways, bio-remediation, engineering microbes for detoxification etc.
-
Synthetic biology: Building DNA circuits, metabolic pathways, genome engineering etc.
Advantages, Limitations & Ethical Considerations
-
Advantages:
-
Precise manipulation of genes.
-
Ability to produce large amounts of protein.
-
Can study gene function in isolation.
-
Wide range of applications (medical, industrial, agricultural).
-
-
Limitations / Challenges:
-
Possible errors in cloning (mutations, incorrect orientation).
-
Expression in heterologous host may not replicate post-translational modifications.
-
Safety concerns (e.g. GMO issues, containment).
-
Technical issues: vector size limitations, expression levels etc.
-
-
Ethical / Regulatory Concerns:
-
Biosafety: release of recombinant organisms, gene flow etc.
-
Ethics of gene therapy/germline modification.
-
Patents/ownership of gene products.
-
Public acceptance/labelling of GM foods, etc.
-