Introduction
- Antibodies, also known as immunoglobulins (Ig), are specialized glycoproteins produced by the immune system in response to foreign substances, called antigens.
- Their primary function is to identify and neutralize harmful invaders such as bacteria, viruses, and toxins.
- Each antibody is uniquely tailored to bind to a specific antigen, and they play a critical role in the body’s defense mechanisms.
- The immune system produces antibodies in response to pathogens, foreign molecules, or even cancerous cells, marking them for destruction or neutralization.
- Antibodies are an essential component of humoral immunity, which is the part of the immune system responsible for producing antibodies to fight infections.
- Antibodies can exist in several different classes, each serving distinct functions, from neutralizing toxins and pathogens to activating other immune system components for a coordinated attack.
- Their ability to recognise and specifically bind to antigens makes them invaluable for both immune defence and diagnostic purposes.
Polyclonal Antibodies
Polyclonal antibodies are a type of antibody that is derived from multiple B cell clones, each recognizing a different epitope (part of the antigen). Unlike monoclonal antibodies, which are produced by a single clone of B cells and recognize one specific epitope, polyclonal antibodies can recognize and bind to multiple epitopes on the same antigen.
Production of Polyclonal Antibodies:
-
Animal Immunization: Polyclonal antibodies are typically produced by immunizing animals (such as rabbits, goats, or sheep) with a specific antigen. The immune system of the animal responds by generating a variety of antibodies against different parts (epitopes) of the antigen.
-
Serum Collection: After the immune response is activated, the animal’s blood is collected, and the serum (which contains the antibodies) is separated. These antibodies are then purified for use in research, diagnostics, or therapeutic applications.
Features:
-
Diversity: Polyclonal antibodies are a mixture of antibodies that target multiple epitopes on a single antigen. This diversity can be beneficial in recognizing and binding to different forms of the same antigen, making polyclonal antibodies more versatile than monoclonal antibodies.
-
Cross-Reactivity: Due to their broad reactivity, polyclonal antibodies may sometimes bind to similar antigens from other organisms or species. This feature can be advantageous in certain diagnostic tests but may also lead to cross-reactivity.
-
Faster Production: Polyclonal antibodies can be produced more quickly and at a lower cost compared to monoclonal antibodies, making them ideal for initial experiments or applications that require a broad response.
Uses of Polyclonal Antibodies:
-
Diagnostic Applications: They are widely used in immunoassays (like ELISA, Western blotting, and immunohistochemistry) to detect pathogens, disease markers, or specific proteins.
-
Therapeutic Applications: Polyclonal antibodies have been used in passive immunization, where antibodies from an immune individual (or animal) are given to another individual to provide temporary protection against infections or toxins.
-
Research: In laboratory research, polyclonal antibodies are commonly used to detect specific proteins, cellular components, or pathogens in samples.
Advantages:
-
Broad Specificity: They can bind to multiple epitopes on an antigen, making them effective for detecting and neutralizing pathogens in various forms.
-
Ease of Production: Polyclonal antibodies are easier and quicker to produce compared to monoclonal antibodies, which require more specialized and time-consuming techniques.
Disadvantages:
-
Batch Variability: Since polyclonal antibodies are derived from multiple B cell clones, different batches of polyclonal antibodies may have slightly different compositions, which can cause variability in results.
-
Less Specificity: Because they bind to several epitopes, polyclonal antibodies may sometimes show cross-reactivity, leading to potential false positives in certain applications.
Development of Antibodies
- The development of antibodies is a crucial part of the body’s immune response to foreign invaders such as bacteria, viruses, and other pathogens.
- Antibodies, also known as immunoglobulins (Ig), are produced by specialized B cells in response to an antigen.
- The process involves complex interactions between B cells, helper T cells, and other immune system components, ensuring the production of antibodies that can specifically target and neutralize pathogens.
Steps Involved in the Development of Antibodies:
-
Antigen Recognition:
-
The development of antibodies begins when a B cell encounters an antigen that it is specific for. B cells have B cell receptors (BCRs) on their surface, which are antibodies bound to the surface of the cell.
-
When the antigen binds to the BCR, it activates the B cell. The antigen could be a pathogen, virus, or foreign substance such as a toxin.
-
-
Antigen Processing and Presentation:
-
After binding to the antigen, the B cell internalizes the antigen and processes it. The antigen is broken down into smaller pieces and then displayed on the surface of the B cell through a process called antigen presentation.
-
The antigen is presented on the MHC (major histocompatibility complex) class II molecules, which are recognized by helper T cells (CD4+).
-
-
Activation by Helper T Cells:
-
The helper T cell recognizes the antigen presented by the B cell and becomes activated.
-
The activated helper T cell releases cytokines (chemical signals) that help further activate the B cell. This interaction is crucial for the full activation of the B cell and for initiating a robust antibody response.
-
-
B Cell Proliferation and Differentiation:
-
Upon activation, the B cell undergoes clonal expansion, which means it rapidly divides to produce multiple copies of itself. These cells are clones of the original B cell, all with the same specificity for the antigen.
-
Some of the B cells differentiate into plasma cells, which are the antibody-producing factories. Other B cells become memory B cells, which provide long-term immunity by “remembering” the specific antigen for future encounters.
-
-
Antibody Production:
-
Plasma cells begin producing large amounts of antibodies (immunoglobulins). These antibodies are secreted into the bloodstream, where they circulate and bind to the antigen.
-
Each antibody produced by a plasma cell is specific to the original antigen. The antibody’s variable region binds to the antigen, marking it for destruction or neutralization.
-
-
Affinity Maturation:
-
During the immune response, affinity maturation occurs. This is a process in which the antibodies produced by B cells improve their ability to bind to the antigen with greater specificity and strength.
-
Somatic hypermutation is a mechanism that introduces random mutations into the variable region of the antibody genes. B cells with higher affinity antibodies are selected to proliferate, enhancing the overall immune response.
-
-
Memory Formation:
-
Memory B cells are long-lived cells that “remember” the antigen. After the infection is cleared, these cells persist in the body and are ready to respond more rapidly if the same antigen is encountered again in the future.
-
Upon re-exposure to the same pathogen, memory B cells can quickly differentiate into plasma cells, producing large amounts of the specific antibody and providing immunity to the individual.
-
Types of Immune Responses:
-
Primary Immune Response:
-
The first time the immune system encounters an antigen, the response is slow because the body needs to activate new B cells, generate antibodies, and form memory cells.
-
Initially, IgM antibodies are produced, followed by IgG antibodies after a few days to weeks.
-
-
Secondary Immune Response:
-
If the body encounters the same antigen again, the memory B cells respond rapidly, producing high levels of IgG antibodies much faster and more effectively.
-
This secondary response is much faster and more robust than the primary response, providing immunity to infections upon re-exposure.
-
Structure of Immunoglobulins
- Immunoglobulins (Ig), commonly known as antibodies, are specialized glycoproteins produced by the immune system to recognize and neutralize foreign invaders, such as bacteria, viruses, and toxins.
- The structure of antibodies is uniquely designed to recognize and bind to specific antigens, which are molecules typically found on the surface of pathogens.
- Antibodies share a common basic structure but differ slightly across their various classes (IgG, IgM, IgA, IgE, and IgD), which allows them to perform specific roles in immune defense.
- Below is a detailed breakdown of the structure of immunoglobulins.
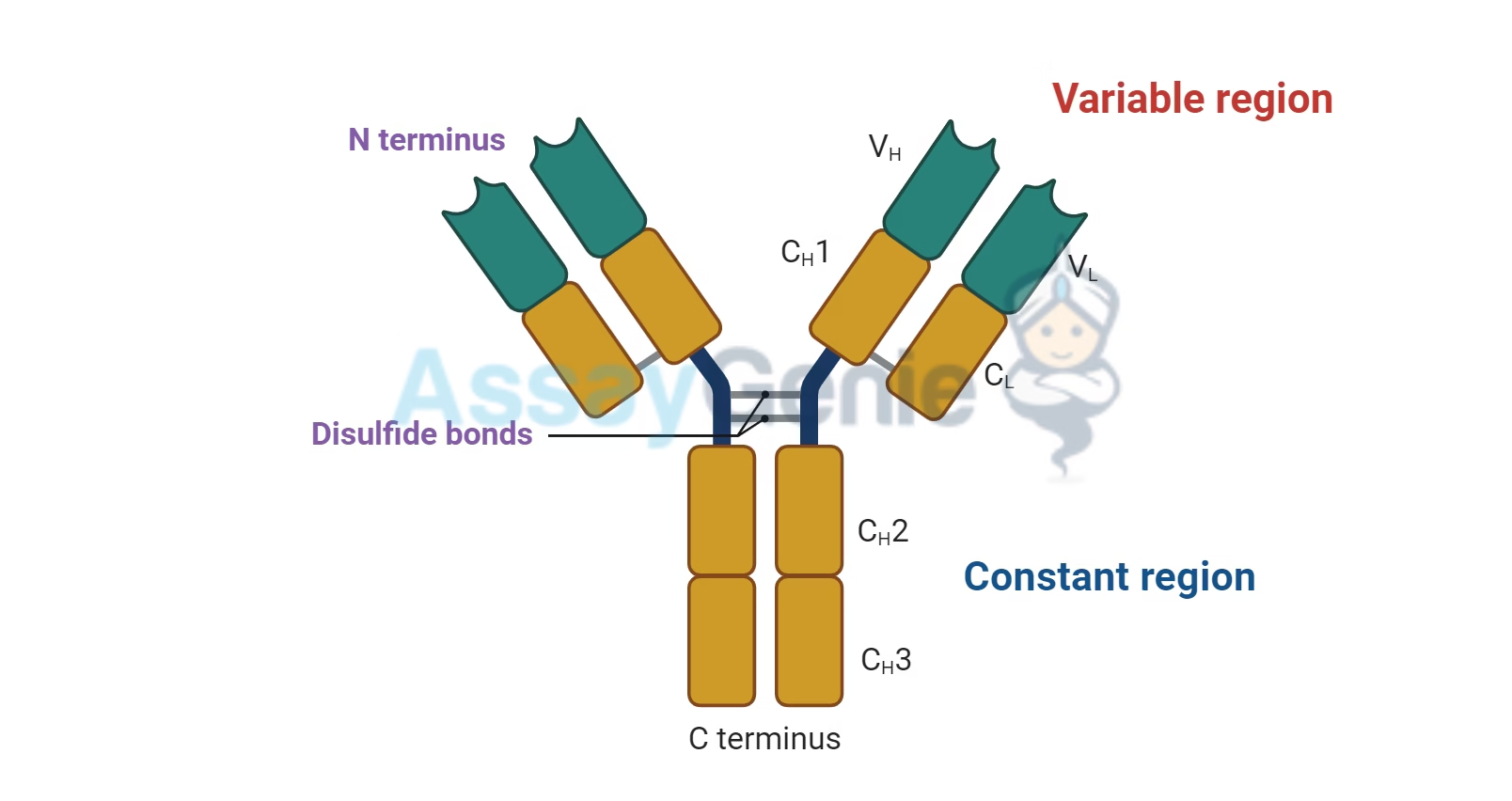
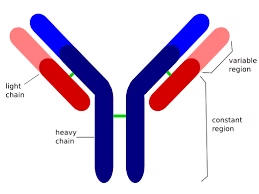
Basic Structure of Antibody
An antibody is Y-shaped and consists of four polypeptide chains:
-
Two heavy chains (H chains)
-
Two light chains (L chains)
These chains are connected by disulfide bonds and held together to form a functional antibody molecule.
Components of Antibody Structure:
-
Heavy Chains (H Chains):
-
Each antibody has two identical heavy chains. These chains are longer than the light chains and are composed of a series of structural domains.
-
The heavy chain determines the antibody’s class (IgG, IgM, IgA, IgE, or IgD). Each class of immunoglobulin has a different heavy chain structure.
-
-
Light Chains (L Chains):
-
Each antibody also has two identical light chains, which are smaller than the heavy chains. There are two types of light chains: kappa (κ) and lambda (λ).
-
Both light chains in an antibody are of the same type (either both kappa or both lambda).
-
-
Variable Regions:
-
The variable regions of both the heavy chains and the light chains are the parts of the antibody that are responsible for antigen binding. These regions are called Fab (Fragment Antigen Binding) regions.
-
The variable regions contain a specific sequence of amino acids that make them highly diverse. This diversity allows antibodies to bind to a wide variety of antigens.
-
-
Constant Regions:
-
The constant regions of both the heavy chains and light chains form the Fc (Fragment Crystallizable) region, which does not change between antibodies of the same class.
-
The constant region of the heavy chain determines the antibody’s isotype (IgG, IgA, IgM, IgE, or IgD) and is involved in the interaction with other components of the immune system, such as phagocytes or complement proteins.
-
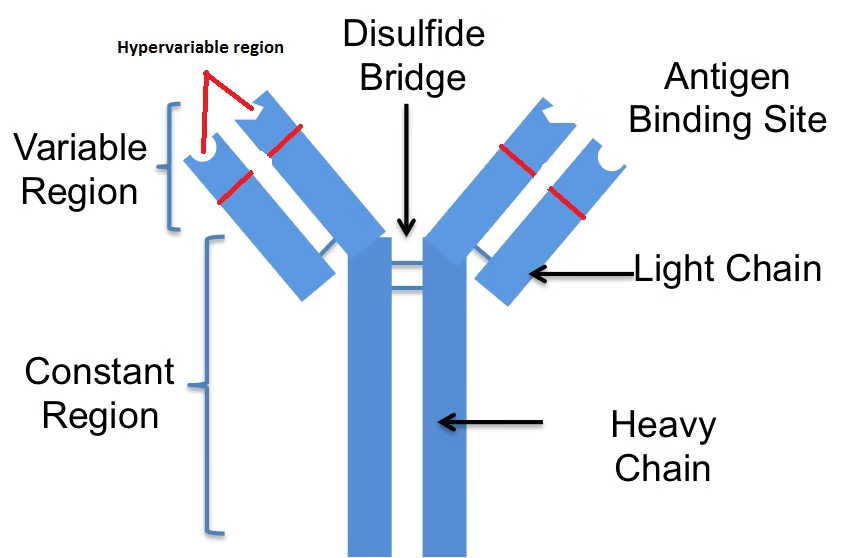
Structure Breakdown
-
Antigen-Binding Region (Fab Region):
-
The Fab region is the portion of the antibody that binds to the antigen. It includes part of the variable region of both the heavy and light chains.
-
The Fab region is responsible for the antibody’s specificity, as the unique sequence of amino acids in the variable regions creates a binding site that fits precisely with a particular antigen, much like a lock and key.
-
The light chains and heavy chains each contribute to the structure of the Fab region, which together form the antigen-binding site.
-
-
Effector Region (Fc Region):
-
The Fc region is the constant part of the antibody that interacts with immune cells (like macrophages, neutrophils, and natural killer cells) through receptors known as Fc receptors.
-
It also binds to complement proteins, which are part of the complement system, an important part of the immune response that helps to destroy pathogens.
-
The Fc region does not bind to the antigen but plays a role in the immune response, including triggering cell-mediated immunity and complement activation.
-
Functional Domains of the Immunoglobulin
An antibody molecule consists of several functional domains that perform specific roles during immune response:
-
Variable Domains:
-
Found in both the light and heavy chains, these domains are responsible for the diversity of antibodies and the ability to specifically bind to an antigen. The variable domains are the most important regions for antigen recognition and binding.
-
-
Constant Domains:
-
These domains are more conserved in structure across different antibodies of the same class and are essential for the antibody’s isotype and effector functions. The constant region’s interaction with immune cells and complement proteins plays a significant role in the antibody’s ability to trigger immune responses.
-
Characteristics of Immunoglobulins
Immunoglobulins (Ig), commonly known as antibodies, are specialized proteins produced by the immune system to identify and neutralize foreign substances like pathogens (bacteria, viruses), toxins, and other harmful molecules. Each class of immunoglobulin has unique structural characteristics and plays a specific role in the immune response.
Characteristics of Immunoglobulins
-
Structure:
-
All immunoglobulins share a Y-shaped structure composed of two heavy chains and two light chains.
-
The variable regions at the tips of the Y bind to specific antigens, and the constant region determines the class of the antibody (IgG, IgM, IgA, IgE, IgD) and interacts with other components of the immune system.
-
-
Specificity:
-
Each antibody is highly specific to the antigen it recognizes. The variability in the variable regions allows antibodies to bind to a vast array of antigens, ensuring the immune system can defend against a wide range of pathogens.
-
-
Diversity:
-
The immune system produces a huge diversity of antibodies, each targeting a unique epitope on an antigen. This diversity is generated by a process called somatic recombination, which allows the body to produce millions of different antibodies.
-
-
Memory:
-
Some antibodies are produced by memory B cells, which “remember” previous infections or exposures. These memory cells provide long-lasting immunity, enabling the body to mount a faster and stronger immune response if the same pathogen is encountered again.
-
-
Half-Life:
-
Antibodies have different half-lives depending on their type. For example, IgG has a relatively long half-life (several weeks), providing long-term immunity, while IgM has a shorter half-life and is typically present during the initial stages of an immune response.
-
Functions of Immunoglobulins
Each class of immunoglobulin has distinct functions that contribute to the immune defense mechanisms in the body. Below is an explanation of the key roles performed by antibodies:
1. Neutralization:
-
Function: Antibodies bind to pathogens (such as viruses or bacteria) or toxins, preventing them from entering or infecting host cells. This process is known as neutralization.
-
Example: IgG antibodies can neutralize viruses by blocking their ability to bind to host cells and replicate.
2. Opsonization:
-
Function: Antibodies can “tag” pathogens for destruction by phagocytes (such as macrophages and neutrophils) in a process called opsonization. The Fc region of the antibody binds to receptors on phagocytic cells, enhancing the ability of these cells to engulf and destroy the pathogen.
-
Example: IgG antibodies play a key role in opsonization by coating the surface of bacteria, making them easier for phagocytes to recognize and consume.
3. Activation of the Complement System:
-
Function: Antibodies, especially IgG and IgM, can activate the complement system—a series of proteins in the blood that enhance the immune response by promoting inflammation, increasing phagocytosis, and directly lysing pathogens.
-
Example: When IgM binds to an antigen, it activates the complement system, leading to the formation of the membrane attack complex (MAC), which can destroy the pathogen’s cell membrane.
4. Antibody-Dependent Cellular Cytotoxicity (ADCC):
-
Function: Antibodies can recruit immune cells, like natural killer (NK) cells, to destroy infected or cancerous cells in a process called antibody-dependent cellular cytotoxicity (ADCC). The antibody binds to the target cell, and its Fc region interacts with receptors on NK cells, triggering the release of cytotoxic substances that kill the target.
-
Example: IgE plays a role in ADCC against parasitic infections, while IgG is involved in killing tumor cells or infected cells through NK cell activation.
5. Immune Response Regulation:
-
Function: Antibodies can also help regulate immune responses. The binding of antibodies to antigens can influence the production of cytokines, signaling molecules that modulate the immune response. Some antibodies, like IgD, are involved in regulating B cell activation.
-
Example: IgD is found on the surface of B cells and plays a role in their activation and differentiation, helping to fine-tune the immune response.
6. Passive Immunity:
-
Function: Antibodies can be transferred from one individual to another to provide temporary protection against pathogens. This is called passive immunity and occurs naturally through the placenta (where IgG is passed from mother to fetus) or through breast milk (IgA).
-
Example: A newborn receives IgG antibodies through the placenta, providing immunity against infections during the first months of life.
Classes of Immunoglobulins
The five classes of immunoglobulins (IgG, IgM, IgA, IgE, IgD) have specific roles in the immune system based on their structure and function. Here’s an overview of each class:
IgG (Immunoglobulin G)
-
Structure: Monomeric (single unit).
-
Function: The most abundant antibody in the blood and extracellular fluid. IgG plays a major role in:
-
Neutralizing pathogens.
-
Opsonization (enhancing phagocytosis).
-
Activating the complement system.
-
Providing long-term immunity after infection or vaccination.
-
Crossing the placenta to provide passive immunity to the fetus.
-
IgM (Immunoglobulin M)
-
Structure: Pentameric (five units).
-
Function: The first antibody produced during a primary immune response. It is very effective at:
-
Activating the complement system.
-
Neutralizing pathogens.
-
Providing early defense against infections.
-
IgM is mainly found in the blood and lymph and is the predominant antibody during the early stages of infection.
-
IgA (Immunoglobulin A)
-
Structure: Dimeric (two units).
-
Function: Found in mucosal areas such as the respiratory, gastrointestinal, and urogenital tracts. IgA:
-
Prevents pathogens from adhering to mucosal surfaces.
-
Neutralizes toxins.
-
Found in secretions such as saliva, tears, and breast milk, providing local immunity.
-
IgA in breast milk provides passive immunity to infants.
-
IgE (Immunoglobulin E)
-
Structure: Monomeric (single unit).
-
Function: Involved in:
-
Allergic reactions: IgE binds to allergens and triggers the release of histamine from mast cells and basophils, leading to symptoms of allergies.
-
Immune responses to parasitic infections: IgE activates immune cells to fight parasites, such as helminths (worms).
-
IgE plays a central role in Type I hypersensitivity reactions (e.g., asthma, hay fever, anaphylaxis).
-
IgD (Immunoglobulin D)
-
Structure: Monomeric (single unit).
-
Function: Found mainly on the surface of B cells. IgD:
-
Helps activate B cells during the initiation of the immune response.
-
Plays a role in the regulation of B cell function, although its exact role is still not fully understood.
-