- Antimicrobial Sensitivity Testing (AST) is crucial for determining the susceptibility of microorganisms (bacteria, fungi) to various antimicrobial agents.
- It helps clinicians choose the right treatment and avoid the overuse of antibiotics, which can lead to resistance.
- Here’s a more detailed explanation of the various AST methods and their applications:
Disk Diffusion Method (Kirby-Bauer Method)
Principle:
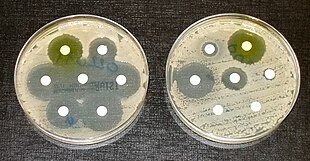
The disk diffusion method is a qualitative test to determine the effectiveness of antimicrobial agents against a microorganism by observing the zone of inhibition around antibiotic-impregnated paper disks placed on an agar plate.
Procedure:
- Preparation: A bacterial suspension (usually from a pure culture) is prepared, and the surface of an agar plate (e.g., Mueller-Hinton agar) is inoculated using a sterile swab to create an even bacterial lawn.
- Disk Placement: Antibiotic-impregnated paper disks are placed on the surface of the inoculated agar.
- Incubation: The plate is incubated at 35-37°C for 18-24 hours.
- Measurement: After incubation, zones of inhibition (clear areas around the disks where bacteria cannot grow) are measured. The diameter of the zone is compared to standardized charts to determine susceptibility.
Interpretation:
- Susceptible (S): The zone of inhibition is large enough to indicate that the antibiotic effectively inhibits the bacteria.
- Resistant (R): A small or no zone of inhibition indicates antibiotic resistance.
- Intermediate (I): A moderate zone of inhibition, suggesting that higher doses or concentrations may be required for treatment.
Advantages:
- Simple and inexpensive: Easy to perform and widely available in clinical laboratories.
- Provides broad-spectrum data: Can test multiple antibiotics simultaneously.
- Standardized interpretation: Clear guidelines for interpreting results based on zone diameters.
Disadvantages:
- Limited to certain organisms: Not suitable for all microorganisms (e.g., anaerobes or fastidious organisms).
- Requires an even bacterial lawn: The inoculum size must be carefully controlled.
Broth Microdilution Method
Principle:
Broth microdilution determines the minimum inhibitory concentration (MIC), the lowest antimicrobial concentration that prevents the microorganism’s visible growth.
Procedure:
- Preparation: Prepare a series of dilutions of the antibiotic in microtiter plates or tubes, with each well containing a different concentration of the antimicrobial agent.
- Inoculation: Inoculate each well with a standardized microbial suspension.
- Incubation: Plates or tubes are incubated at 35-37°C for 18-24 hours.
- Assessment: After incubation, the MIC is determined by observing the lowest concentration of the antibiotic where no visible growth is seen (i.e., no turbidity).
Interpretation:
- The MIC is the lowest concentration of the antimicrobial agent that prevents visible bacterial growth.
- The result helps clinicians determine whether an organism is susceptible, intermediate, or resistant to the antibiotic at specific concentrations.
Advantages:
- Quantitative: Provides precise information on the MIC, which is useful for determining the appropriate therapeutic dose.
- Suitable for various organisms: Can be used for bacteria and fungi.
- The gold standard for certain organisms: Especially important in antifungal testing and dealing with resistant pathogens.
Disadvantages:
- Labor-intensive and time-consuming: Requires much manual work and incubation time.
- Requires specialized equipment: Microtiter plates or automated systems are needed to perform the test.
E-Test (Epsilometer Test)
Principle:
The E-test combines the disk diffusion method and broth dilution to determine the MIC. It uses a plastic strip with a gradient of antibiotic concentrations and measures the MIC where the zone of inhibition intersects the strip.
Procedure:
- Preparation: A bacterial lawn is inoculated onto the surface of an agar plate.
- E-test Strip Application: An E-test strip containing an antibiotic gradient is placed on the inoculated plate.
- Incubation: The plate is incubated at 35°C for 18-24 hours.
- Interpretation: After incubation, the MIC is read at the point where the zone of inhibition intersects the E-test strip.
Interpretation:
- The MIC is the point where the zone of inhibition intersects with the strip. This is a continuous gradient, so it gives more precise MIC results than the disk diffusion method.
Advantages:
- Combination of methods: E-test provides a numerical MIC value and visual zone of inhibition, offering qualitative and quantitative data.
- Less labor-intensive: Easier and faster than broth dilution methods, providing accurate results.
- Versatile: Can be used with various organisms (bacteria and fungi).
Disadvantages:
- More expensive: E-test strips are costlier than simple paper disks.
- Requires accurate measurements: The interpretation of MIC values depends on the precise reading of the zone of inhibition.
Agar Dilution Method
Principle:
The agar dilution method is similar to broth dilution but involves incorporating different concentrations of antimicrobial agents directly into the agar medium. The MIC is determined by observing the lowest concentration that prevents microbial growth.
Procedure:
- Preparation: Prepare agar plates with different concentrations of the antimicrobial agent.
- Inoculation: A standardized bacterial suspension is inoculated on the surface of the agar plates.
- Incubation: The plates are incubated at 35-37°C for 18-24 hours.
- Observation: After incubation, the MIC is the lowest antibiotic concentration where growth is completely inhibited.
Interpretation:
- The MIC is determined based on the concentration of antibiotic that prevents bacterial growth.
Advantages:
- Accurate MIC determination: Provides a clear and reliable MIC value for determining the most effective antibiotic.
- Can be used for a wide range of organisms: Works for both bacteria and fungi.
Disadvantages:
- Time-consuming: More labor-intensive than disk diffusion and E-test.
- Requires specialized equipment: Involves preparing multiple agar plates with different antibiotic concentrations.
Automated Systems (e.g., VITEK 2, Microscan)
Principle:
Automated systems use pre-filled test cards or microplates containing different antimicrobial agents and automatically measure the MIC. These systems can identify organisms and test their susceptibility simultaneously.
Procedure:
- Inoculation: A standardized microbial suspension is added to a pre-filled test card or microplate containing various antibiotics.
- Automatic Reading: The system incubates the plate and automatically reads the MIC and susceptibility results.
- Data Analysis: The system provides a report with the interpretation of results.
Advantages:
- Rapid results: Provides quick results (usually within hours), helping in decision-making.
- High-throughput: Automated systems can handle large numbers of samples simultaneously.
- Accuracy: Eliminates human error in reading results, increasing consistency.
Disadvantages:
- Expensive: The systems require significant investment in equipment and maintenance.
- Requires specialized training: Operators must be trained to use automated systems effectively.
Interpretation of Results and Breakpoints
- Breakpoints: Standardized values established by organizations like the Clinical and Laboratory Standards Institute (CLSI) or the European Committee on Antimicrobial Susceptibility Testing (EUCAST). These values define the concentration ranges for susceptibility (S), intermediate (I), and resistance (R) for each antimicrobial agent.
- Minimum Inhibitory Concentration (MIC): The MIC is the lowest concentration of an antibiotic that completely inhibits bacterial growth. MIC values guide therapy, with lower MICs indicating stronger antibiotic efficacy.
Interpretation of MIC values:
- Susceptible: The organism will likely respond to treatment with the given antibiotic.
- Resistant: The organism is unlikely to respond to the antibiotic at standard dosages.
- Intermediate: The organism may respond to the antibiotic at higher doses, or the treatment may need to be adjusted.
Factors Influencing AST Results
- Inoculum Size: The number of microorganisms inoculated into the test must be consistent to ensure accurate results. Too many bacteria can cause falsely resistant results, and too few may lead to false susceptibility.
- Antibiotic Concentrations: Standard concentrations must be used, as varying concentrations can alter the interpretation of results.
- Incubation Conditions: Temperature, time, and atmosphere can all affect microbial growth and the effectiveness of antimicrobial agents. For example, some bacteria may need anaerobic conditions or extended incubation times.
- Media Composition: The type of agar or broth used can influence the antibiotic’s activity, affecting the test’s outcome.
Clinical Applications of AST
- Guiding Therapy: AST results help clinicians choose the most effective antimicrobial for treating infections, particularly in severe or resistant cases.
- Monitoring Resistance: AST is a key tool in surveillance programs to monitor antimicrobial resistance (AMR) trends and guide public health interventions.
- Optimizing Dosing: By determining the MIC, AST helps determine the correct dosing regimen to ensure the drug reaches an effective concentration in the body.