- Assaying body fluids involves detecting and quantifying various analytes, including cells, proteins, metabolites, electrolytes, hormones, and microorganisms.
- These tests are performed to diagnose diseases, monitor therapeutic responses, and assess metabolic functions.
- The following provides a deeper dive into common assay methods for analyzing body fluids.
Enzyme-Linked Immunosorbent Assay (ELISA)
Principle:
ELISA detects the presence of specific antibodies or antigens in a sample. The method uses an enzyme-labeled antibody that reacts with a substrate, generating a measurable signal, typically a color change.
Types of ELISA:
- Direct ELISA: The antigen is immobilized on the plate, and a labeled antibody specific to the antigen is used for detection.
- Indirect ELISA: An unlabeled primary antibody binds to the antigen, and a labeled secondary antibody detects the primary antibody.
- Sandwich ELISA: The capture and detection antibodies are used to “sandwich” the antigen between them. This is the most commonly used format for antigen detection.
- Competitive ELISA: A known amount of antigen competes with the antigen in the sample for antibody binding. The more antigens in the sample, the less color development occurs.
Procedure:
- Antigens or antibodies are bound to a solid phase (often a microplate).
- Body fluid is added, and specific binding interactions (antigen-antibody) can occur.
- A secondary enzyme-labeled antibody is added and binds to the complex.
- The enzyme reacts with a substrate, producing a color change, measured using a spectrophotometer.
Applications:
- Detection of infections (e.g., HIV, Hepatitis).
- Hormone quantification (e.g., thyroid hormones, cortisol).
- Autoimmune disease diagnostics (e.g., rheumatoid factor).
- Cancer biomarker detection (e.g., prostate-specific antigen).
Radioimmunoassay (RIA)
Principle:
RIA uses radioactively labeled molecules, typically antibodies or antigens, to detect small amounts of substances in a sample. The bound radiolabeled antibody or antigen is measured using a scintillation counter.
Procedure:
- A sample is incubated with a known amount of radiolabeled antibody or antigen.
- The bound complex is separated from free radiolabeled substances after incubation.
- The radioactivity of the bound complex is measured, and the concentration of the analyte is determined based on standard curves.
Applications:
- Detection of hormones (e.g., thyroid, growth hormone).
- Measurement of drug levels in serum.
- Quantification of vitamins and small molecules.
Chemiluminescent Immunoassay (CLIA)
Principle:
CLIA uses chemiluminescence to detect antigen-antibody reactions. The enzyme-labeled antibody produces light when it reacts with a substrate, and the amount of light is proportional to the concentration of the analyte.
Procedure:
- The body fluid is incubated with an enzyme-labeled antibody specific to the target molecule.
- The enzyme reacts with a substrate to generate light, measured by a luminometer.
Applications:
- Detection of viral infections (e.g., HIV, COVID-19).
- Quantification of cytokines, cardiac markers, and hormones.
Fluorescent Immunoassay (FIA)
Principle:
FIA uses fluorescent-labeled antibodies that emit light upon excitation by a specific wavelength. The intensity of the emitted light is directly proportional to the amount of antigen in the sample.
Procedure:
- The sample is incubated with a fluorescently labeled antibody.
- After binding, the sample is exposed to ultraviolet light, and the fluorescence emitted is measured.
Applications:
- Detection of antibodies in autoimmune diseases.
- Quantification of hormones, such as insulin or cortisol.
Spectrophotometry
Principle:
Spectrophotometry is based on the principle that molecules absorb light at specific wavelengths. The absorption of light by the sample is measured, and this absorbance is proportional to the concentration of the analyte.
Procedure:
- A sample (e.g., serum or urine) is exposed to light of a specific wavelength.
- The amount of light absorbed by the sample is measured, which is used to determine the concentration of the analyte.
Applications:
- Measure blood or urine glucose, cholesterol, bilirubin, and creatinine.
- Enzyme assays, such as ALT or AST, to monitor liver function.
- Serum protein analysis.
Gas Chromatography-Mass Spectrometry (GC-MS)
Principle:
GC-MS is a technique used for separating and identifying compounds in a sample. Gas chromatography separates volatile compounds, and mass spectrometry provides structural identification of the separated components.
Procedure:
- A sample is injected into a chromatographic column, where components are separated based on their volatility and interaction with the column.
- The mass spectrometer analyzes the separated components, which detects their mass-to-charge ratio.
Applications:
- Drug and toxin detection (e.g., illicit drugs, alcohol, pesticides).
- Monitoring volatile biomarkers (e.g., in breath for metabolic disorders).
- Quantifying metabolites (e.g., steroids, fatty acids, vitamins).
High-Performance Liquid Chromatography (HPLC)
Principle:
HPLC separates compounds in a liquid sample based on their interactions with a stationary phase inside a column, while a mobile phase carries the sample through the column.
Procedure:
- A sample is injected into a liquid chromatograph and passes through a column packed with a stationary phase.
- The sample components are separated and eluted at different times based on their interactions with the stationary phase.
- The eluted compounds are detected by UV absorption, fluorescence, or conductivity.
Applications:
- Detection of drugs and pharmaceuticals in body fluids.
- Quantification of amino acids, vitamins, and lipids in blood or urine.
- Detection of metabolic biomarkers (e.g., glucose, fatty acids).
Polymerase Chain Reaction (PCR)
Principle:
PCR is a molecular method that amplifies small quantities of DNA or RNA to detectable levels. This method is highly sensitive and can detect the genetic material of pathogens or mutations in body fluids.
Procedure:
- DNA or RNA is extracted from the sample.
- Specific primers are added to the sample to amplify the target DNA or RNA region.
- The reaction mixture is subjected to thermal cycling, which repeats the amplification process.
- The amplification is detected either by gel electrophoresis or fluorescent dyes.
Applications:
- Detection of bacterial and viral infections (e.g., COVID-19, tuberculosis).
- Identification of genetic mutations (e.g., in cancer diagnostics or genetic disorders).
- Quantification of RNA viruses (e.g., HIV viral load testing).
Urine Dipstick Test
Principle:
The urine dipstick test is a qualitative test that detects specific substances in urine based on chemical reactions. Reagent pads on a strip change color when exposed to certain analytes.
Procedure:
- The urine sample is applied to the reagent strip.
- The strip contains pads impregnated with chemicals that react with substances in the urine, producing a color change.
- The color change is compared to a chart, indicating specific analyte concentrations.
Applications:
- Routine screening for glucose (diabetes), protein (kidney function), and hemoglobin (urinary tract infections).
- Detection of pH, ketones (diabetic ketoacidosis), and bilirubin (liver dysfunction).
- Screening for urinary tract infections (e.g., nitrites, leukocyte esterase).
Blood Gas Analysis (ABG)
Principle:
ABG analysis measures the concentrations of oxygen (pO₂), carbon dioxide (pCO₂), bicarbonate (HCO₃⁻), and pH to assess respiratory and metabolic functions.
Procedure:
- An arterial blood sample is obtained from the radial or femoral artery.
- The sample is analyzed for oxygenation status, carbon dioxide levels, and pH.
- Results are used to diagnose respiratory acidosis/alkalosis, metabolic acidosis, or alkalosis.
Applications:
- Monitoring respiratory function in critically ill patients or those on ventilators.
- Assessing the metabolic status (e.g., in patients with diabetes or kidney failure).
- Diagnosing acid-base imbalances (e.g., in pneumonia, sepsis, or drug overdose).
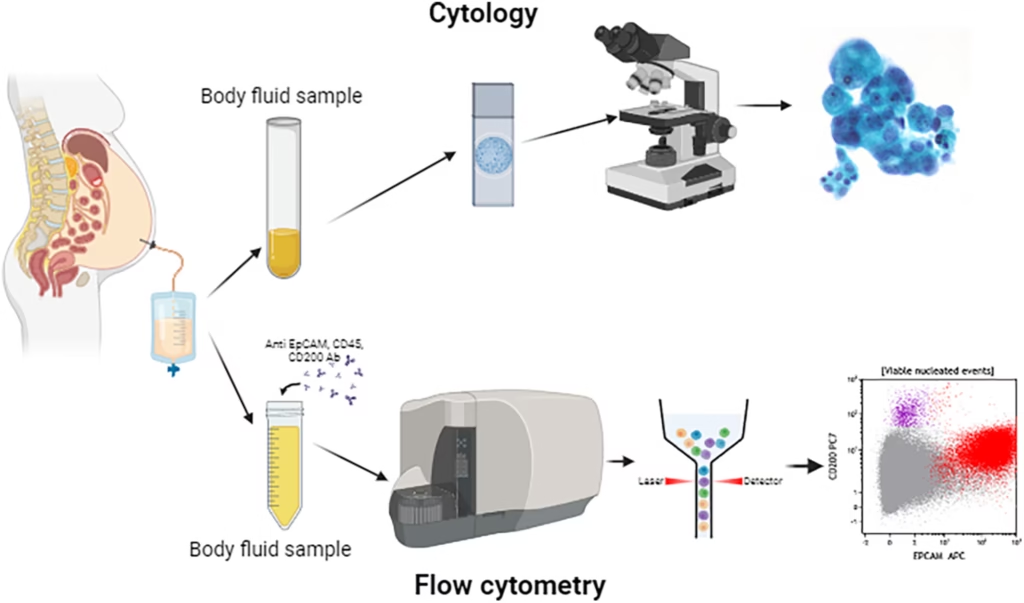
Cytological and Microscopic Examination
Principle:
Microscopic examination allows direct visualization of cells, microorganisms, or crystals in body fluids. Staining techniques enhance visibility and help identify specific pathogens or abnormalities.
Procedure:
- A sample is placed on a microscope slide, and a few drops of stain (e.g., Gram stain, Wright-Giemsa) are applied.
- The slide is examined under a microscope for cells, microorganisms (bacteria, fungi, viruses), or crystals.
Applications:
- Diagnosis of infections (e.g., bacterial or fungal cultures in CSF, pleural fluid).
- Detection of cancer cells (e.g., pleural fluid cytology).
- Identification of crystals in synovial fluid (e.g., gout, pseudogout).