
Introduction
- Fats (lipids) are essential biomolecules that serve as a major source of energy in the human body.
- They provide 9 kcal/g of energy, making them the most energy-dense nutrients.
- Biosynthesis of fats (lipogenesis) occurs when excess carbohydrates and proteins are converted into fatty acids and stored as fat.
- Fat synthesis mainly takes place in the liver, adipose tissue, and lactating mammary glands.
- Synthesized fatty acids are esterified with glycerol to form triacylglycerols (TAGs).
- Triacylglycerols are the principal form in which fats are stored.
- Storage of fats occurs primarily in adipose tissue within specialized cells called adipocytes.
- Stored fats act as a long-term energy reserve and are mobilized during fasting, exercise, or stress.
- Fat storage also provides thermal insulation and mechanical protection to vital organs.
- The processes of fat synthesis and storage are hormonally regulated, mainly by insulin, glucagon, and epinephrine.
Biosynthesis of Fats (Lipogenesis)
Lipogenesis is the process by which fatty acids and triacylglycerols (TAGs) are synthesized from excess carbohydrates and stored as fat in the body.
Site
-
Cytosol
-
Mainly in:
-
Liver
-
Adipose tissue
-
Lactating mammary gland
-
Main Components
Lipogenesis has two steps:
-
Fatty acid synthesis
-
Triacylglycerol (TAG) synthesis
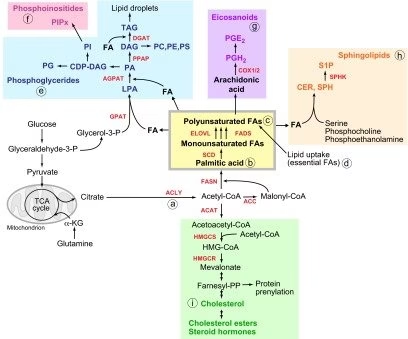
Fatty Acid Biosynthesis (De Novo Lipogenesis)
Fatty acid biosynthesis is the de novo synthesis of long-chain saturated fatty acids from acetyl-CoA in the cytosol, mainly during the fed state when carbohydrate intake is high.
1. Site of Synthesis
- Cytosol
- Major tissues:
- Liver (primary site)
- Adipose tissue
- Lactating mammary gland
2. Sources of Carbon and Reducing Power
- Acetyl-CoA – carbon units
- Malonyl-CoA – chain elongation donor
- NADPH – reducing agent
Sources of NADPH
- Pentose phosphate pathway (HMP shunt)
- Malic enzyme (malate → pyruvate)
3. Transport of Acetyl-CoA (Citrate Shuttle)
Acetyl-CoA is formed in mitochondria but synthesis occurs in cytosol.
Steps:
- Acetyl-CoA + oxaloacetate → citrate (mitochondria)
- Citrate transported to cytosol
- Citrate → acetyl-CoA + oxaloacetate
(enzyme: ATP-citrate lyase)
4. Steps in Fatty Acid Biosynthesis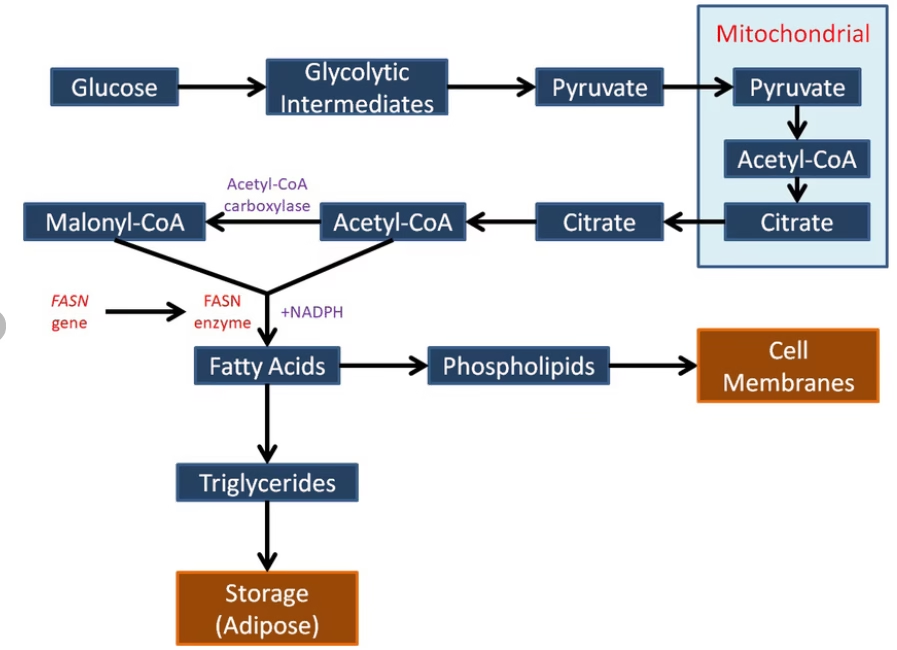
5. Regulation of Fatty Acid Biosynthesis
A. Allosteric Regulation
- Citrate → activates ACC
- Palmitoyl-CoA → inhibits ACC
B. Hormonal Regulation
- Insulin → activates ACC (dephosphorylation)
- Glucagon & epinephrine → inhibit ACC (phosphorylation)
C. Nutritional Regulation
- High carbohydrate diet → ↑ lipogenesis
- Fasting → ↓ lipogenesis
Triacylglycerol (TAG) Synthesis
Triacylglycerol (TAG) synthesis is the biochemical process by which three fatty acids are esterified to a glycerol backbone, forming TAG—the principal storage form of fat in the body.
1. Sites of TAG Synthesis
- Liver
- Adipose tissue
- Intestinal mucosa
- Lactating mammary gland
2. Precursors Required
- Fatty acyl–CoA
- Glycerol-3-phosphate
Source of Glycerol-3-Phosphate
- Liver:
- From glycerol (via glycerol kinase)
- From glycolysis (DHAP → glycerol-3-phosphate)
- Adipose tissue:
- From glycolysis only
(adipose tissue lacks glycerol kinase)
- From glycolysis only
3. Steps of TAG Synthesis (Glycerol Phosphate Pathway)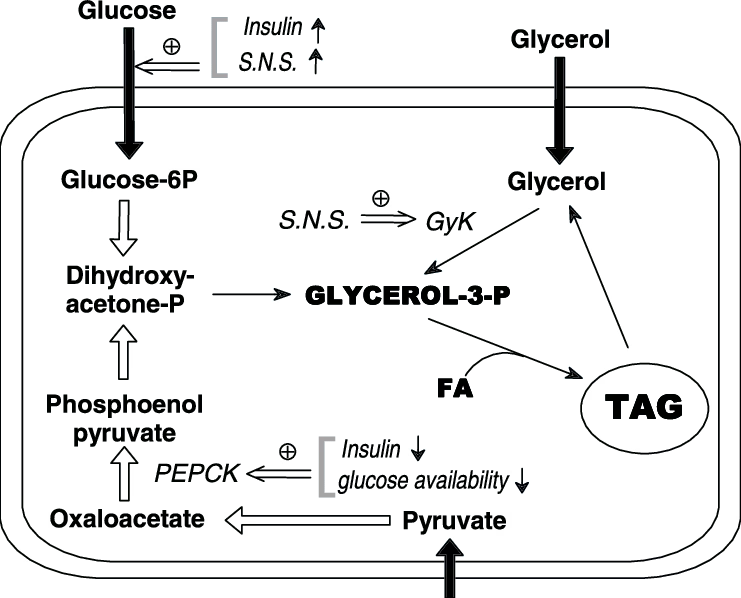
Step 1: Formation of Lysophosphatidic Acid
- Glycerol-3-phosphate + fatty acyl-CoA
- Enzyme: Glycerol-3-phosphate acyltransferase (GPAT)
Step 2: Formation of Phosphatidic Acid
- Lysophosphatidic acid + fatty acyl-CoA
- Enzyme: 1-acylglycerol-3-phosphate acyltransferase
Step 3: Formation of Diacylglycerol (DAG)
- Phosphatidic acid → DAG
- Enzyme: Phosphatidic acid phosphatase (Lipin)
Step 4: Formation of Triacylglycerol
- DAG + fatty acyl-CoA → TAG
- Enzyme: Diacylglycerol acyltransferase (DGAT)
4. Intestinal TAG Synthesis (Dietary Fat)
- Occurs in intestinal mucosal cells
- Uses monoacylglycerol pathway
- TAGs packaged into chylomicrons
- Transported via lymphatics → blood
5. Fate of Synthesized TAG
- Liver:
- Packaged into VLDL
- Transported to adipose tissue & muscle
- Adipose tissue:
- Stored as fat droplets
- Intestine:
- Transported as chylomicrons
6. Regulation of TAG Synthesis
Hormonal Regulation
- Insulin:
- ↑ glucose uptake
- ↑ glycerol-3-phosphate
- ↑ TAG synthesis
- Glucagon / Epinephrine:
- ↓ TAG synthesis
- ↑ lipolysis
Nutritional State
- Fed state → TAG synthesis ↑
- Fasting state → TAG synthesis ↓
7. Storage of TAG
- Stored in adipocytes as lipid droplets
- Coated by perilipin
- Mobilized by hormone-sensitive lipase (HSL) during fasting
8. Clinical & Exam Significance
- Excess TAG synthesis → obesity
- Increased hepatic TAG → fatty liver
- Overproduction of VLDL → hypertriglyceridemia
- Insulin resistance → increased TAG accumulation
Storage of Fats
Storage of fats refers to the accumulation of triacylglycerols (TAGs) within specialized cells called adipocytes, primarily in adipose tissue, serving as the body’s major long-term energy reserve.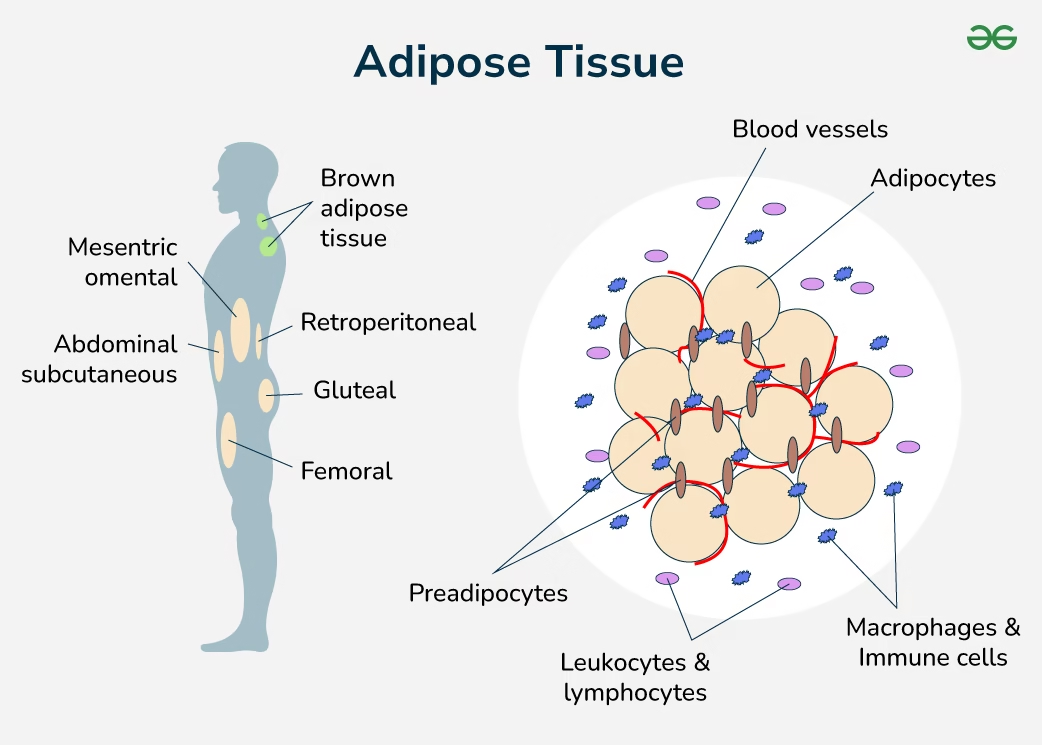
1. Major Site of Fat Storage
 Adipose tissue
Adipose tissue
- Subcutaneous fat
- Visceral fat (omentum, mesentery, perirenal)
Minor storage also occurs in liver and skeletal muscle, but excess storage here is pathological.
2. Form in Which Fat Is Stored
- Stored as triacylglycerols (TAGs)
- Located in cytoplasmic lipid droplets
3. Structure of Stored Fat (Adipocyte)
- Single large lipid droplet (unilocular)
- Surrounded by a phospholipid monolayer
- Associated regulatory proteins:
- Perilipin – protects TAG from lipase action
- CGI-58 (activator of lipolysis)
4. Types of Adipose Tissue
A. White Adipose Tissue (WAT)
- Primary site of TAG storage
- Functions:
- Energy storage
- Insulation
- Mechanical cushioning
- Endocrine activity
B. Brown Adipose Tissue (BAT)
- Rich in mitochondria
- Contains UCP-1 (thermogenin)
- Generates heat (non-shivering thermogenesis)
- Prominent in infants and to a lesser extent in adults
5. Hormonal Regulation of Fat Storage
Fed State
- ↑ Insulin
- ↑ Glucose uptake (GLUT-4)
- ↑ Glycerol-3-phosphate
- ↑ TAG synthesis
- ↓ Lipolysis
Fasting / Stress State
- ↑ Glucagon, epinephrine
- Activation of hormone-sensitive lipase (HSL)
- TAG → free fatty acids + glycerol
- Fatty acids released into blood (bound to albumin)
6. Mobilization vs Storage Balance
- Storage favored in fed state
- Mobilization favored in fasting, exercise, stress
- Controlled by perilipin phosphorylation
7. Physiological Importance of Fat Storage
- High-energy yield (9 kcal/g)
- Thermal insulation
- Protection of vital organs
- Source of essential fatty acids
- Carrier of fat-soluble vitamins (A, D, E, K)
8. Endocrine Functions of Adipose Tissue
Adipose tissue acts as an endocrine organ by secreting:
- Leptin – appetite regulation
- Adiponectin – insulin sensitivity
- Resistin, TNF-α – metabolic regulation
9. Clinical Significance
- Excess storage → obesity
- Visceral fat → ↑ cardiovascular risk
- Ectopic fat storage → insulin resistance
- Impaired mobilization → dyslipidemia
- Fatty liver due to excess TAG accumulation
Hormonal Regulation of Fat Storage
- Hormonal regulation of fat storage ensures a balance between energy storage (fed state) and energy mobilization (fasting/stress state).
- This regulation mainly occurs in adipose tissue and is mediated through changes in enzyme activity and phosphorylation status.
1. Hormones Involved
- Insulin – anabolic, promotes fat storage
- Glucagon – catabolic, promotes fat mobilization
- Epinephrine (Adrenaline) – rapid fat mobilization
- Cortisol – permissive, long-term effects on fat metabolism
- Growth hormone – anti-insulin effect on adipose tissue
2. Insulin: Promoter of Fat Storage (Fed State)
Source
- β-cells of pancreas
Mechanisms
- ↑ Glucose uptake into adipocytes (GLUT-4)
- ↑ Glycolysis → ↑ glycerol-3-phosphate
- ↑ TAG synthesis
- ↑ Lipoprotein lipase (LPL) activity in adipose tissue
→ uptake of fatty acids from chylomicrons & VLDL - ↓ Hormone-sensitive lipase (HSL) activity
Net Effect
- Increased fat storage
- Decreased lipolysis
3. Glucagon: Promoter of Fat Mobilization (Fasting State)
Source
- α-cells of pancreas
Mechanisms
- Activates adenylate cyclase
- ↑ cAMP → PKA activation
- Phosphorylation of:
- HSL → active
- Perilipin → exposes lipid droplet
Net Effect
- Decreased fat storage
- Increased lipolysis
4. Epinephrine: Stress-Induced Lipolysis
Source
- Adrenal medulla
Mechanisms
- Binds β-adrenergic receptors
- ↑ cAMP → PKA activation
- Strong activation of HSL
- Rapid release of free fatty acids
Role
- Provides immediate energy during:
- Exercise
- Stress
- Hypoglycemia
5. Cortisol: Permissive Hormone
Effects
- Enhances lipolytic action of glucagon & epinephrine
- Increases availability of fatty acids
- Chronic excess → central obesity
6. Growth Hormone
- Anti-insulin action in adipose tissue
- ↓ glucose uptake
- ↑ lipolysis
- Spares glucose for brain
7. Role of Perilipin in Hormonal Control
- Unphosphorylated perilipin:
- Protects TAG from lipase action
- Phosphorylated perilipin:
- Allows HSL access to lipid droplets
MCQs
1. Lipogenesis mainly occurs in which cellular compartment?
A. Mitochondria
B. Lysosomes
C. Cytosol
D. Nucleus
2. The major organ responsible for fatty acid synthesis is:
A. Kidney
B. Liver
C. Brain
D. Spleen
3. The primary carbon source for fatty acid synthesis is:
A. Pyruvate
B. Acetyl-CoA
C. Malate
D. Citrate
4. Acetyl-CoA is transported from mitochondria to cytosol in the form of:
A. Malate
B. Oxaloacetate
C. Citrate
D. Pyruvate
5. The enzyme that converts citrate back to acetyl-CoA in cytosol is:
A. Citrate synthase
B. ATP-citrate lyase
C. Acetyl-CoA dehydrogenase
D. Malic enzyme
6. The rate-limiting enzyme of fatty acid synthesis is:
A. Fatty acid synthase
B. Acetyl-CoA carboxylase
C. Thiokinase
D. Carnitine acyltransferase
7. Acetyl-CoA carboxylase requires which cofactor?
A. FAD
B. NAD⁺
C. Biotin
D. Thiamine
8. The immediate product formed by acetyl-CoA carboxylase is:
A. Palmitate
B. Acetoacetate
C. Malonyl-CoA
D. Citrate
9. Fatty acid synthase is best described as:
A. Single enzyme
B. Mitochondrial enzyme
C. Multifunctional enzyme complex
D. Membrane-bound protein
10. The final product of de novo fatty acid synthesis is:
A. Stearic acid
B. Oleic acid
C. Palmitic acid
D. Linoleic acid
11. The reducing power required for fatty acid synthesis is supplied by:
A. NADH
B. FADH₂
C. NADPH
D. ATP
12. A major source of NADPH for lipogenesis is:
A. TCA cycle
B. Pentose phosphate pathway
C. Glycolysis
D. β-oxidation
13. Fatty acid synthesis occurs predominantly in which nutritional state?
A. Fasting
B. Starvation
C. Fed state
D. Exercise
14. Which hormone stimulates fatty acid synthesis?