Introduction
-
Buffer solutions are special solutions that resist changes in pH when small amounts of acid or base are added.
-
They are composed of a weak acid and its conjugate base, or a weak base and its conjugate acid.
-
The main function of a buffer is to maintain a constant hydrogen ion concentration (pH) in a solution, even when external factors tend to change it.
-
Buffer solutions play a crucial role in biological and chemical systems, where enzymes and reactions require a specific pH to function optimally.
-
Common examples include the phosphate buffer, acetate buffer, and bicarbonate buffer, which help maintain pH stability in blood, cells, and laboratory experiments.
-
The capacity of a buffer depends on the concentration of its components and the ratio between acid and base forms.
Types of Buffer Solutions
-
Acidic Buffer Solution:
-
Maintains an acidic pH (below 7).
-
Formed by mixing a weak acid with the salt of that acid and a strong base.
-
Example: Acetic acid (CH₃COOH) and sodium acetate (CH₃COONa) maintain a pH of around 4.75.
-
-
Basic Buffer Solution:
-
Maintains a basic or alkaline pH (above 7).
-
Prepared by combining a weak base with its salt formed with a strong acid.
-
Example: Ammonium hydroxide (NH₄OH) and ammonium chloride (NH₄Cl) — maintains pH around 9.25.
-
-
Neutral Buffer Solution:
-
Maintains a neutral pH (around 7).
-
Usually made from neutral salts of weak acids and weak bases.
-
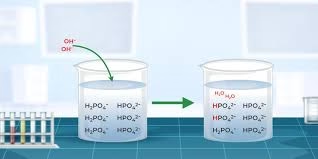
Example: A potassium dihydrogen phosphate (KH₂PO₄) and disodium hydrogen phosphate (Na₂HPO₄) mixture maintains pH near 7.0.
-
-
Biological Buffer Solution:
-
Found naturally in living systems to maintain constant physiological pH.
-
Example: Bicarbonate buffer system (H₂CO₃/HCO₃⁻) in blood maintains pH around 7.4.
-
-
Mixed Buffer System:
-
Contains more than one buffer pair and can resist pH changes over a wider range.
-
Example: Phosphate buffer (NaH₂PO₄ and Na₂HPO₄) used in laboratory and biological experiments.
-
Mechanism
-
Buffer mechanism works on the principle of the common ion effect — the presence of a common ion suppresses the ionization of a weak acid or base, helping the solution resist pH changes.
-
When a small amount of acid (H⁺) is added to a buffer, the conjugate base present in the buffer combines with these hydrogen ions to form the weak acid, preventing a large increase in H⁺ concentration.
Example: In acetic acid–sodium acetate buffer:
H⁺ + CH₃COO⁻ → CH₃COOH -
When a small amount of base (OH⁻) is added, the weak acid in the buffer reacts with it to form water and its conjugate base, minimizing any rise in pH.
Example: CH₃COOH + OH⁻ → CH₃COO⁻ + H₂O
-
In a basic buffer like NH₄OH/NH₄Cl, the mechanism is similar:
-
Added acid (H⁺) reacts with NH₄OH → NH₄⁺ + H₂O
-
Added base (OH⁻) reacts with NH₄⁺ → NH₃ + H₂O
-
-
In this way, a buffer maintains nearly constant pH by neutralizing small additions of acid or base, keeping the ratio of weak acid to conjugate base nearly constant.
Preparation of Buffer Solutions
-
Identify the Required pH Range:
Decide whether an acidic or basic buffer is needed based on the desired pH of the solution.-
For pH < 7 → Acidic buffer
-
For pH > 7 → Basic buffer
-
-
Select Appropriate Components:
-
For an acidic buffer, choose a weak acid and its salt with a strong base (e.g., CH₃COOH and CH₃COONa).
-
For a basic buffer, choose a weak base and its salt with a strong acid (e.g., NH₄OH and NH₄Cl).
-
-
Calculate Required Molar Ratios:
Use the Henderson–Hasselbalch equation to determine the correct ratio of acid to salt (or base to salt) for the desired pH:pH=pKa+log[Salt] / [Acid]
or
pOH=pKb+log[Salt] / [Base]
-
Prepare Stock Solutions:
Prepare separate standard (0.1 M or 1 M) solutions of the weak acid/base and its salt using distilled water. -
Mix in Correct Proportion:
Measure the calculated volumes of both components using a pipette or burette and mix them in a clean volumetric flask. -
Check and Adjust pH (if necessary):
Use a pH meter to measure the solution’s pH. Adjust slightly by adding small amounts of acid or base until the desired pH is achieved. -
Make Up to Final Volume:
Dilute the mixture to the required final volume with distilled water and mix thoroughly to ensure uniformity. -
Label and Store:
Transfer the prepared buffer into a clean, airtight bottle, label it with composition and pH, and store it properly to avoid contamination or CO₂ absorption.
Example:
Preparation of 0.1 M Phosphate Buffer (pH 7.4):
- Select the pKa:
- Phosphoric acid has multiple pKa values, but for this buffer, the relevant pKa is 7.2, close to the desired pH of 7.4.
- Henderson-Hasselbalch Equation:
Ph = pKa + log ([Base]/[Acid])
- Since the desired pH (7.4) is slightly higher than the pKa (7.2), the base (Na₂HPO₄) concentration will be slightly higher than the acid (NaH₂PO₄).
- Calculation of Molar Ratio:
- The Henderson-Hasselbalch equation gives:
7.4 = 7.2 + log ([Base]/[Acid])
Solving this gives a base-to-acid ratio of approximately 1.6:1.
- Mixing:
- Prepare 0.1 M solutions of Na₂HPO₄ and NaH₂PO₄.
- Mix 61.5 mL of 0.1 M Na₂HPO₄ with 38.5 mL of 0.1 M NaH₂PO₄.
- Adjust Volume:
- Add distilled water to bring the total volume to 100 ml.
- Check pH:
- Use a pH meter to check the buffer’s pH. Adjust slightly with small amounts of NaOH or HCl if necessary to keep the pH to 7.4.
Applications
-
In Biological Systems:
Buffer solutions help maintain a constant pH in body fluids like blood and intracellular fluids, which is vital for enzyme activity and metabolic processes.
Example: The bicarbonate buffer system maintains blood pH around 7.4. -
In Pharmaceutical Preparations:
Buffers are used in medicines and injections to maintain the required pH for stability and proper absorption of drugs.
Example: Phosphate buffers are used in ophthalmic and intravenous preparations. -
In Chemical and Biochemical Laboratories:
Buffers are essential in titrations, electrophoresis, chromatography, and enzyme assays where precise pH control is required.
Example: Tris buffer and phosphate buffer are used in enzyme kinetics experiments. -
In Industrial Processes:
Used in fermentation, dyeing, tanning, and electroplating industries where controlled pH is necessary for optimal reactions. -
In Food Industry:
Buffers maintain the taste, texture, and shelf life of foods by preventing unwanted pH changes.
Example: Citric acid and sodium citrate buffer used in soft drinks and dairy products. -
In Agriculture:
Buffer solutions are used to calibrate pH meters and to maintain soil pH for plant growth studies. -
In Analytical Chemistry:
Buffers provide a stable pH environment for accurate chemical analysis, especially in colorimetric and spectrophotometric assays. -
In Molecular Biology and Biotechnology:
Commonly used in DNA extraction, PCR reactions, and protein purification to maintain enzyme activity and structural integrity of biomolecules.
MCQs
-
A buffer solution resists changes in:
A. Temperature
B. pH
C. Concentration
D. Pressure -
A buffer is generally made of:
A. Strong acid and strong base
B. Weak acid and its salt
C. Strong acid and its salt
D. Neutral compounds -
Which of the following is an acidic buffer?
A. NH₄OH + NH₄Cl
B. CH₃COOH + CH₃COONa
C. Na₂HPO₄ + NaH₂PO₄
D. HCl + NaCl -
Which of the following is a basic buffer?
A. NH₄OH + NH₄Cl
B. H₂CO₃ + NaHCO₃
C. CH₃COOH + CH₃COONa
D. H₃PO₄ + NaH₂PO₄ -
The pH of an acidic buffer is:
A. Greater than 7
B. Equal to 7
C. Less than 7
D. Cannot be determined -
The Henderson–Hasselbalch equation is used to:
A. Calculate pH of a buffer
B. Calculate temperature of a reaction
C. Measure molar concentration
D. Determine ionic strength -
The Henderson–Hasselbalch equation for an acidic buffer is:
A. pH = pKa + log [acid]/[salt]
B. pH = pKa + log [salt]/[acid]
C. pH = pKb + log [base]/[salt]
D. pOH = pKa + log [salt]/[acid] -
The buffer capacity depends on:
A. Volume of buffer
B. Ratio of acid to base
C. Total concentration of buffer components
D. Both B and C -
The pKa of acetic acid is 4.75. When [acid] = [salt], the pH of buffer is:
A. 2.5
B. 4.75
C. 7.0
D. 9.0 -
Which of the following maintains blood pH?
A. Phosphate buffer
B. Acetate buffer
C. Bicarbonate buffer
D. Ammonium buffer -
The bicarbonate buffer system maintains pH around:
A. 6.8
B. 7.0
C. 7.4
D. 8.2 -
In a buffer, if acid concentration is doubled, pH will:
A. Increase
B. Decrease
C. Remain same
D. Be zero -
Which of the following pairs is not a buffer system?
A. H₂CO₃ / NaHCO₃
B. NH₄OH / NH₄Cl
C. HCl / NaCl
D. CH₃COOH / CH₃COONa -
What happens when a small amount of acid is added to a buffer?
A. pH increases sharply
B. pH decreases sharply
C. pH remains nearly constant
D. Solution becomes neutral -
In a basic buffer, the weak base reacts with:
A. Added acid (H⁺)
B. Added base (OH⁻)
C. Salt ions
D. None of these -
In an acidic buffer, the weak acid reacts with:
A. Added OH⁻ ions
B. Added H⁺ ions
C. Both
D. Neither -
Which of the following is a neutral buffer?
A. NaH₂PO₄ + Na₂HPO₄
B. HCl + NaCl
C. NH₄OH + NH₄Cl
D. CH₃COOH + CH₃COONa -
Which of the following maintains the pH of intracellular fluids?
A. Bicarbonate buffer
B. Phosphate buffer
C. Acetate buffer
D. Tris buffer -
Buffers are important in biochemical reactions because:
A. They speed up reactions
B. They maintain optimal pH for enzyme activity
C. They reduce ionic strength
D. They act as catalysts -
A buffer solution has maximum capacity when:
A. pH = pKa
B. pH = 7
C. pH > pKa
D. pH < pKa -
Which of the following combinations gives a buffer of pH ≈ 4.75?
A. CH₃COOH + CH₃COONa
B. NH₄OH + NH₄Cl
C. NaH₂PO₄ + Na₂HPO₄
D. H₂CO₃ + NaHCO₃ -
Buffer solutions are used in:
A. Enzyme assays
B. Blood pH control
C. Pharmaceutical formulations
D. All of these -
The buffering action fails when:
A. Large amounts of acid/base are added
B. pKa changes
C. Concentration becomes zero
D. All of these -
Which of the following buffers is commonly used in biological experiments?
A. Tris buffer
B. Borate buffer
C. Glycine buffer
D. All of these -
pH of a buffer solution mainly depends on:
A. Temperature
B. Ratio of salt to acid/base
C. Total volume
D. Color of solution -
The common ion effect plays a key role in:
A. Buffer mechanism
B. Redox reaction
C. Electrolysis
D. Combustion -
Which buffer system is used in the human kidney for acid-base balance?
A. Ammonium buffer
B. Phosphate buffer
C. Bicarbonate buffer
D. Both A and B -
The term “buffer capacity” refers to:
A. Ability to change color
B. Ability to resist pH change
C. Amount of acid/base neutralized
D. Both B and C -
When equal moles of acid and salt are mixed, the buffer shows:
A. Maximum buffering capacity
B. Minimum buffering capacity
C. No buffering action
D. Unstable pH -
Buffers are used in calibration of:
A. Colorimeter
B. pH meter
C. Spectrophotometer
D. Centrifuge
Answer Key
-
B
-
B
-
B
-
A
-
C
-
A
-
B
-
D
-
B
-
C
-
C
-
B
-
C
-
C
-
A
-
A
-
A
-
B
-
B
-
A
-
A
-
D
-
A
-
D
-
B
-
A
-
D
-
D
-
A
-
B