
Definition
- Carbohydrates are biomolecules consisting of carbon (C), hydrogen (H), and oxygen (O), typically following the empirical formula (CH2O)n, where “n” represents the number of carbon atoms.
- Carbohydrates can be defined as aldehyde or ketone derivatives of polyhydric alcohols.
- Most carbohydrates taste sweet, and hence they are popularly known as sugars.
- Carbohydrates are essential for energy storage, structural support, and many metabolic processes in living organisms.
Properties of Carbohydrates
- Chemical Composition:
- Made up of carbon (C), hydrogen (H), and oxygen (O) atoms, generally in the ratio of 1:2:1.
- Solubility:
- Simple carbohydrates (monosaccharides and some disaccharides) are generally water-soluble because they have hydroxyl (-OH) groups, which form hydrogen bonds with water molecules.
- Complex carbohydrates (polysaccharides) vary in solubility; some, like cellulose, are insoluble due to their large size and structure.
- Sweetness:
- Simple sugars like glucose and fructose taste sweet, while complex carbohydrates like starch and cellulose lack sweetness.
- Reducing and Non-reducing Properties:
- Reducing sugars (e.g., glucose and lactose) have free aldehyde or ketone groups to participate in redox reactions.
- Non-reducing sugars (e.g., sucrose) lack free aldehyde or ketone groups and do not act as reducing agents.
- Polymerization:
- Carbohydrates can polymerise to form complex molecules. For instance, glucose molecules can link to form starch, glycogen, or cellulose, each with distinct properties and functions.
- Structural Variability:
- Carbohydrates exhibit structural diversity, allowing them to form linear, branched, or highly complex chains, contributing to their function versatility.
Functions of Carbohydrates
- Primary Energy Source:
- Carbohydrates are the main source of energy for living organisms. During digestion, carbohydrates are broken down into glucose, which cells use to produce ATP (adenosine triphosphate) through cellular respiration.
- Example: Glucose is quickly absorbed and provides immediate energy, which is particularly crucial for brain function and physical activities.
- Energy Storage:
- Polysaccharides like starch (in plants) and glycogen (in animals) are stored energy reserves.
- Example: Glycogen in the liver and muscles can be rapidly broken down into glucose to maintain blood sugar levels during fasting or exercise.
- Structural Support:
- Some carbohydrates provide structural integrity to cells and tissues.
- Example: Cellulose, a polysaccharide found in plant cell walls, gives rigidity and support to plants. Chitin, another polysaccharide, forms the exoskeleton of insects and crustaceans.
- Cell Recognition and Signalling:
- Carbohydrates attached to proteins and lipids on cell surfaces (glycoproteins and glycolipids) play critical roles in cell recognition, signalling, and immune response.
- Example: Blood group antigens on red blood cells are carbohydrate structures determining blood type and compatibility.
- Protection and Lubrication:
- Some carbohydrates serve protective roles in the body or function as lubricants.
- Example: Mucus, which contains glycoproteins, protects internal body surfaces (such as the respiratory and digestive tracts) and lubricates tissues.
- Fiber and Digestive Health:
- Indigestible carbohydrates, such as dietary fiber (mainly cellulose), help maintain digestive health by adding bulk to stool, promoting regular bowel movements, and preventing constipation.
- Example: Fiber-rich foods like whole grains, fruits, and vegetables contribute to gut health and reduce the risk of various digestive disorders.
- Osmotic Balance:
- Carbohydrates play a role in osmotic balance in cells, helping to regulate water movement in and out of cells.
- Example: In plants, the carbohydrate sucrose is involved in osmoregulation, ensuring proper cell water uptake.
Classification of Carbohydrates
Carbohydrates are classified into three major types based on the number of sugar units they contain:
- Monosaccharides – Simple sugars, single-unit carbohydrates.
- Disaccharides – Composed of two monosaccharide units.
- Polysaccharides – Complex carbohydrates made of many sugar units.
Monosaccharides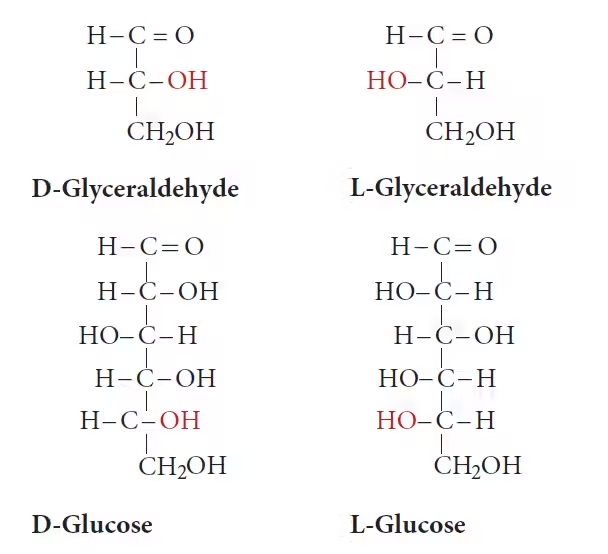
Monosaccharides are the simplest and most basic form of carbohydrates. They are single sugar molecules that cannot be hydrolyzed (broken down) into simpler sugar units. They are the building blocks for all other types of carbohydrates.
Structure and Types of Monosaccharides
Monosaccharides can be classified based on: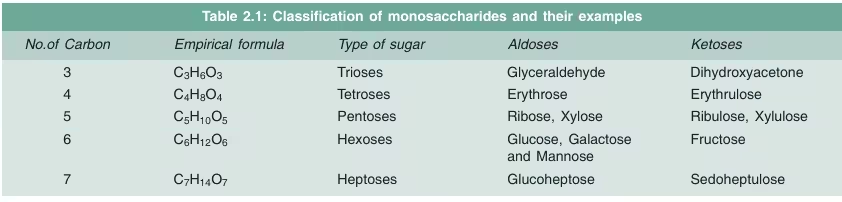
- The number of carbon atoms:
- Trioses: 3 carbon atoms (e.g., glyceraldehyde).
- Pentoses: 5 carbon atoms (e.g., ribose, found in RNA).
- Hexoses: 6 carbon atoms (e.g., glucose, fructose).
- The type of functional group:
- Aldoses: Contain an aldehyde group (-CHO). Example: Glucose.
- Ketoses: Contain a ketone group (-CO). Example: Fructose.
Examples of Monosaccharides:
- Glucose: The most important hexose sugar in human metabolism. Glucose is the primary energy source for most living organisms and is crucial for cellular respiration.
- Sources: Found in fruits, honey, and as part of many carbohydrates.
- Fructose: Known as the sweetest naturally occurring sugar, fructose is commonly found in fruits and honey.
- Sources: Fruits, honey, root vegetables.
- Galactose: Less sweet than glucose and fructose, galactose is part of lactose, the sugar found in milk.
- Sources: Milk and dairy products (as part of lactose).

- Sources: Milk and dairy products (as part of lactose).
Functions of Monosaccharides:
- Immediate energy source: Monosaccharides like glucose are easily absorbed into the bloodstream and provide quick energy for cellular functions.
- Building blocks for more complex carbohydrates: Monosaccharides are the foundational units synthesizing disaccharides and polysaccharides.
- Role in cellular communication: Some monosaccharides are involved in cell recognition and signaling, forming part of glycoproteins and glycolipids in cell membranes.
Disaccharides
- Disaccharides comprise two monosaccharide units linked by a glycosidic bond (a covalent bond formed through dehydration).
- They are a common form of carbohydrate found in many foods.
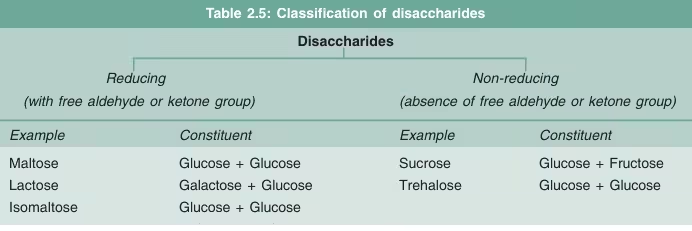
Examples of Disaccharides:
- Sucrose (Glucose + Fructose):
- Common name: Table sugar.
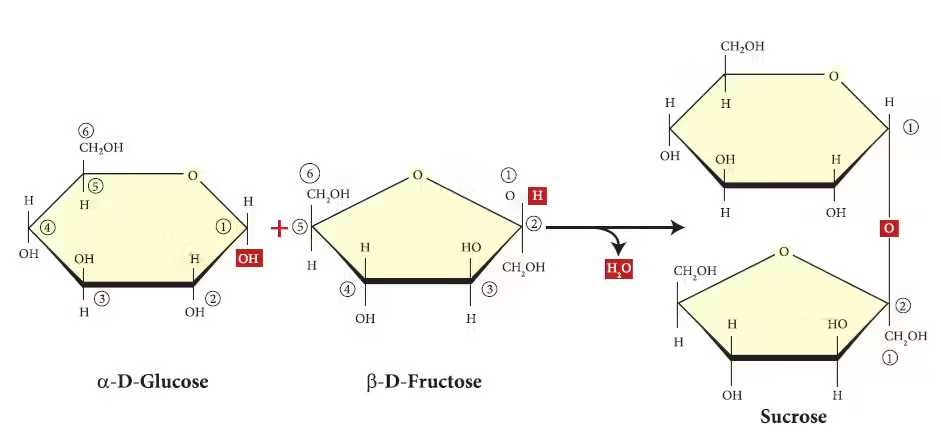
- Sources: Found naturally in plants like sugarcane and sugar beet.
- Function: Major dietary source of energy. When consumed, sucrose is broken down into glucose and fructose for absorption.
- Common name: Table sugar.
- Lactose (Glucose + Galactose):
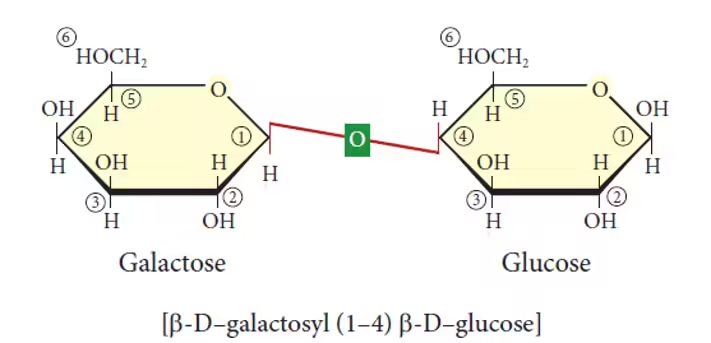
- Common name: Milk sugar.
- Sources: Found in milk and dairy products.
- Function: Provides energy for infants and mammals. It requires the enzyme lactase for digestion, and lactose intolerance occurs when lactase production is insufficient.
- Maltose (Glucose + Glucose):
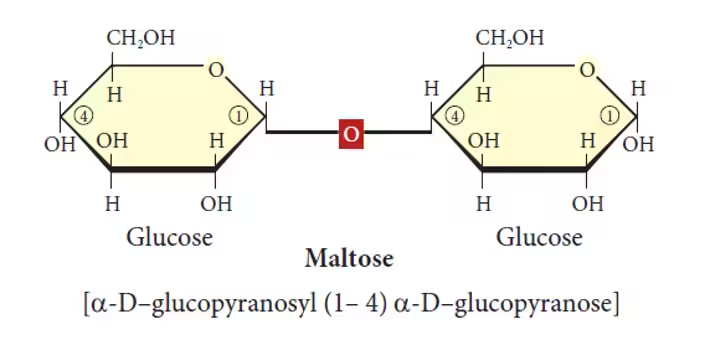
- Common name: Malt sugar.
- Sources: Formed during the breakdown of starch, found in germinating grains and malted products like beer and cereals.
- Function: An intermediate in the digestion of starch, maltose is broken down into glucose molecules for energy.
Functions of Disaccharides:
- Energy source: Like monosaccharides, disaccharides are broken down into their component sugars during digestion, providing energy.
- Transportable form: In plants, disaccharides like sucrose are transported through the phloem to various parts of the plant as energy storage.
- Serve as sweeteners: Disaccharides like sucrose are used as sweeteners in food products due to their taste and energy content.
Polysaccharides
- Polysaccharides are large, complex carbohydrates composed of long chains of monosaccharide units linked by glycosidic bonds.
- These biomolecules are essential for energy storage, structural support, and other critical biological functions in living organisms.
- They are one of the primary classes of macromolecules in nature, alongside proteins, lipids, and nucleic acids.
- Polysaccharides can be classified into two main categories: storage polysaccharides and structural polysaccharides, based on their function and structure.
- They are made up of repeating units of simple sugars (monosaccharides) like glucose, fructose, or galactose.
Classification of Polysaccharides
-
Homopolysaccharides:
These are polysaccharides made from only one type of monosaccharide unit. They include storage and structural polysaccharides.
Examples –
-
-
-
Starch
-
Glycogen
-
Cellulose
-
Chitin
-
Inulin
-
-
- Heteropolysaccharides:
These are polysaccharides made from more than one type of monosaccharide unit. They often have specialised roles, such as in cell signalling or forming complex biomaterials.
Examples –
-
-
-
Agar
-
Gums (e.g., Guar gum, Xanthan gum)
-
Hyaluronic Acid
-
Glycosaminoglycans (e.g., Chondroitin sulfate, Heparin)
-
Pectin
-
-
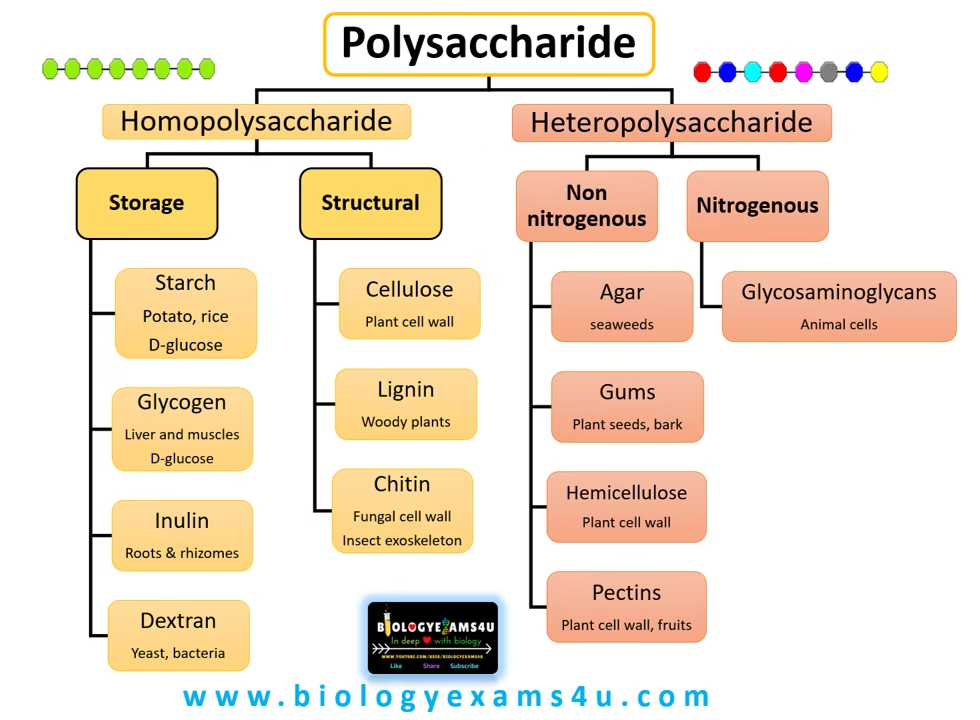
Polysaccharides can be broadly classified into storage and structural polysaccharides.
1. Storage Polysaccharides
Storage polysaccharides are complex carbohydrates that serve as energy reserves. These polysaccharides can be broken down into simpler sugars when the organism requires energy.
Starch
-
Function:
Starch is the primary energy storage molecule in plants. When plants need energy or carbon for growth, starch is broken down into its glucose subunits by enzymes. It acts as a carbohydrate reservoir in plant tissues. -
Structure:
Starch consists of two types of glucose polymers:-
Amylose: A linear polymer of glucose molecules connected by α-1,4-glycosidic bonds. It forms a helical structure and is less soluble in water.
-
Amylopectin: A branched polymer of glucose, where branches occur at α-1,6-glycosidic bonds. It is more soluble and rapidly broken down than amylose.
-
-
Examples:
-
Potatoes, rice, wheat, corn, and legumes are common sources of starch.
-
In the human body, starch is broken down by the enzyme amylase into glucose.
-
Glycogen
-
Function:
Glycogen is the main storage polysaccharide in animals. It serves as a quick-release source of glucose when energy is needed, especially in muscles and the liver. When the body requires energy, glycogen is broken down into glucose molecules through glycogenolysis. -
Structure:
Glycogen is highly branched, consisting of glucose units connected by α-1,4-glycosidic bonds with branching occurring at α-1,6-glycosidic bonds. Its highly branched structure allows for rapid breakdown and energy release. -
Examples:
-
Found in the liver (used for blood sugar regulation) and muscles (used during physical activity).
-
The glycogen stores are limited, and once depleted, the body relies on fat stores for energy.
-
Galactogen
-
Function:
Galactogen is a lesser-known polysaccharide that consists mainly of galactose and is sometimes associated with storage functions in certain organisms like bacteria and seaweeds. It serves as an energy reservoir in some marine organisms and bacteria. -
Structure:
Composed primarily of galactose units, galactogen is often used by marine algae and certain microorganisms as a storage carbohydrate. -
Examples:
Found in certain marine algae and some bacteria. It’s not as common in plants or animals as starch or glycogen.
Inulin
-
Function:
Inulin is a polysaccharide that serves as an energy reserve in some plants. It is particularly found in plants that grow in harsh conditions, where it serves as a source of stored energy during periods of stress. -
Structure:
Inulin is a polymer of fructose units linked by β-2,1-glycosidic bonds. Unlike starch, it is not composed of glucose and cannot be digested by humans, though it has a prebiotic effect in the gut. -
Examples:
Found in the roots and rhizomes of plants such as chicory, Jerusalem artichoke, and onions. It is also found in dandelions and asparagus.
2. Structural Polysaccharides
Structural polysaccharides provide structural integrity to cells, tissues, and organisms. They are not used for energy storage but rather contribute to the mechanical strength and protection of cells.
Arabinoxylans
-
Function:
Arabinoxylans are a type of hemicellulose, a polysaccharide found in the cell walls of plants. They contribute to the rigidity and strength of plant cell walls and provide structural support, particularly in cereal grains. -
Structure:
Composed of a backbone of xylose units with arabinosyl side chains. The structure varies between different plant species, influencing its physical properties and its role in plant architecture. -
Examples:
Found in the cell walls of grasses, wheat, barley, and other cereal grains.
Cellulose
-
Function:
Cellulose is the most abundant organic polymer on Earth. It forms the primary component of plant cell walls and provides structural support. It is extremely strong and resistant to hydrolysis, contributing to the mechanical strength of plants. -
Structure:
Cellulose is made of β-D-glucose units linked by β-1,4-glycosidic bonds. These bonds form straight, unbranched chains that aggregate into bundles known as microfibrils, which provide rigidity to plant cell walls. -
Examples:
-
Found in wood, cotton, paper, and fiber plants. Humans cannot digest cellulose due to the lack of enzymes that can break β-1,4-glycosidic bonds (it’s considered dietary fiber).
-
Chitin
-
Function:
Chitin is a structural polysaccharide found in the exoskeletons of arthropods (such as insects, crustaceans), and in the cell walls of fungi. It provides a rigid structure and acts as a protective barrier. -
Structure:
Composed of N-acetylglucosamine units, which are derivatives of glucose. The units are linked by β-1,4-glycosidic bonds, forming long chains that aggregate into fibers. -
Examples:
Found in the exoskeletons of insects, crustaceans (e.g., crabs, shrimp), and the cell walls of fungi (e.g., mushrooms).
Pectins
-
Function:
Pectins are complex polysaccharides that play a role in plant cell wall integrity, particularly in the middle lamella, where they act as a cementing material that binds plant cells together. Pectins also contribute to the texture and ripening of fruits. -
Structure:
Pectins are composed mainly of galacturonic acid units with varying amounts of other sugars, such as arabinose and xylose. These units are linked through α-1,4-glycosidic bonds. -
Examples:
-
Found in fruits, especially in the peels and cell walls of citrus fruits (oranges, lemons), apples, and berries. Used in the production of jam and jellies due to their gelling properties.
-
Functions of Polysaccharides:
-
Energy Storage
-
Starch and glycogen serve as energy reservoirs. When energy is needed, these polysaccharides are broken down into simpler sugars, which are used in cellular respiration to produce ATP (energy).
-
Starch is the primary form of energy storage in plants, while glycogen is the primary storage form in animals.
-
-
Structural Support
-
Polysaccharides like cellulose and chitin provide structural strength to organisms.
-
Cellulose forms the cell walls in plants, providing rigidity and preventing excessive water loss.
-
Chitin provides support to the exoskeletons of arthropods and other organisms like fungi.
-
-
Lubrication and Tissue Repair
-
Hyaluronic acid is a polysaccharide that serves as a lubricant, reducing friction in joints and other body tissues. It also plays a role in tissue hydration, wound healing, and tissue repair.
-
-
Cell Signaling and Recognition
-
Polysaccharides attached to proteins or lipids (glycoproteins and glycolipids) are involved in cell signaling and recognition. They help determine blood types, immune responses, and facilitate communication between cells.
-
-
Protection
-
Heparin, another specialized polysaccharide, plays a key role in preventing blood clotting. It works by inhibiting clot formation and ensuring that blood flows smoothly.
-