
Introduction
- Chromatography is an important biophysical technique that enables the separation, identification, and purification of the components of a mixture for qualitative and quantitative analysis.
- The Russian botanist Mikhail Tswett coined the term chromatography in
- The first analytical use of chromatography was described by James and Martin in 1952 for using gas chromatography to analyze fatty acids.
- A wide range of chromatographic procedures uses differences in size, binding affinities, charge, and other properties to separate materials.
- It is a powerful separation tool used in all branches of science and is often the only means of separating components from complex mixtures.
Principle of Chromatography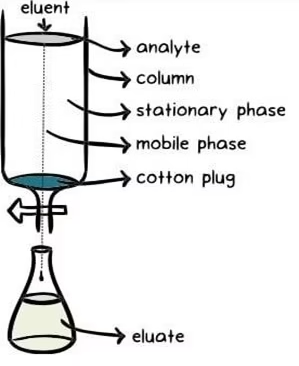
- Chromatography is based on the principle where molecules in a mixture are applied onto the surface or into the solid, and the fluid stationary phase (stable phase) separates from each other while moving with a mobile phase.
- The factors effective in this separation process include molecular characteristics related to adsorption (liquid-solid), partition (liquid-solid), and affinity or differences among their molecular weights.
- Because of these differences, some components of the mixture stay longer in the stationary phase. They move slowly in the chromatography system, while others pass rapidly into the mobile phase and leave the system faster.
Three components thus form the basis of the chromatography technique.
- Stationary phase: This phase is always composed of a “solid” phase or “a layer of a liquid adsorbed on the surface solid support”.
- Mobile phase: This phase is always composed of “liquid” or a “gaseous ”
- Separated molecules
Paper Chromatography
- A chromatography technique that uses paper sheets or strips as the adsorbent, being the stationary phase through which a solution is made to pass is called paper chromatography.
- It is an inexpensive method of separating dissolved chemical substances by their different migration rates across the sheets of paper.
- It is a powerful analytical tool that uses very small quantities of material. Paper chromatography was discovered by Synge and Martin in the year 1943.
Paper Chromatography Principle
- The principle involved can be partition chromatography or adsorption chromatography.
- Partition chromatography because the substances are partitioned or distributed between liquid phases.
- The two phases are water held in the pores of the filter paper, and the other phase is a mobile phase that passes through the paper.
- When the mobile phase moves, the separation of the mixture takes place.
- The compounds in the mixture separate themselves based on the differences in their affinity towards stationary and mobile phase solvents under the capillary action of pores in the paper.
- Adsorption chromatography between solid and liquid phases, wherein the solid surface of the paper is the stationary phase, and the liquid phase is the mobile phase.
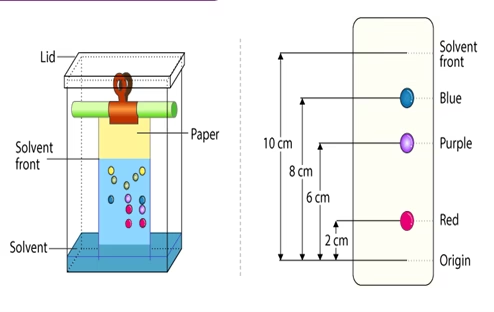
Paper Chromatography Procedure
Below, we have explained the procedure to conduct a Paper Chromatography Experiment for easy understanding students.
- Selecting a suitable type of development: It is decided based on the complexity of the solvent, paper, mixture, etc. Usually, ascending type or radial paper chromatography is used as they are easy to perform. Also, it is easy to handle, the chromatogram obtained is faster, and the process is less time-consuming.
- Selecting a suitable filter paper: The selection of filter paper is done based on the size of the pores and the sample quality.
- Prepare the sample: Sample preparation includes the dissolution of the sample in a suitable solvent (inert with the sample under analysis) used in making the mobile
- Spot the sample on the paper: Samples should be spotted properly using a capillary tube.
- Chromatogram development: Chromatogram development is spotted by immersing the paper in the mobile phase. Due to the capillary action of paper, the mobile phase moves over the sample on the paper.
- Paper drying and compound detection: Once the chromatogram is developed, the paper is dried using an air dryer. Also, the detecting solution can be sprayed on the chromatogram-developed paper and dried to identify the sample chromatogram
Paper Chromatography Applications
There are various applications of paper chromatography. Some of the uses of Paper Chromatography in different fields are discussed below:
- To study the process of fermentation and
- To check the purity of
- To inspect
- To detect the
- To detect the contaminants in drinks and
- To examine the reaction mixtures in biochemical
- To determine dopes and drugs in humans and
Types of paper chromatography:
- Ascending Paper Chromatography – The technique goes with its name as the solvent moves upward.
- Descending Paper Chromatography – The movement of solvent flow due to gravitational pull and capillary action is downwards, hence descending paper chromatography.
- Ascending – Descending Paper Chromatography – In this version of paper chromatography, the movement of solvent occurs in two directions after a particular. Initially, the solvent travels upwards on the paper, which is folded over a rod, and after crossing the rod, it continues with its travel in the downward direction.
- Radial or Circular Paper Chromatography – The sample is deposited at the center of the circular filter paper. Once the spot is dried, the filter paper is tied horizontally on a Petri dish that contains the solvent.
- Two-Dimensional Paper Chromatography – Substances that have the same values can be resolved with the help of two-dimensional paper chromatography.
Thin Layer Chromatography
Thin Layer Chromatography is a technique used to isolate non-volatile mixtures. The experiment is conducted on a sheet of aluminium foil, plastic, or glass coated with a thin layer of adsorbent material. The material usually used is aluminium oxide, cellulose, or silica gel.
On completion of the separation, each component appears as spots separated vertically. Each spot has a retention factor (Rf) expressed as:
Rf = dist. travelled by sample / dist. travelled by solvent
Thin Layer Chromatography Principle
- Like other chromatographic techniques, thin-layer chromatography (TLC) depends on the separation principle.
- The separation relies on the relative affinity of compounds towards both phases.
- The mobile phase compounds move over the stationary phase’s surface.
- The movement occurs so that the compounds with a higher affinity to the stationary phase move slowly, while the other compounds travel fast.
- Therefore, the separation of the mixture is attained.
- On completion of the separation process, the individual components from the mixture appear as spots at respective levels on the plates.
- Suitable detection techniques identify their character and nature.
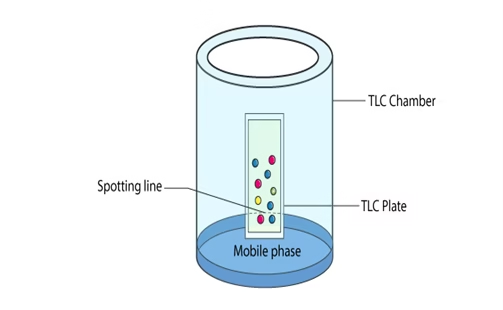
Thin Layer Chromatography Procedure
Before starting with the Thin Layer Chromatography Experiment, let us understand the components required to conduct the procedure and the phases involved.
- Thin Layer Chromatography Plates – ready-made plates that are chemically inert and stable are used. The stationary phase is applied on its surface as a thin layer. The stationary phase on the plate has a fine particle size and a uniform thickness.
- Thin Layer Chromatography Chamber – The chamber is used to develop plates. It is responsible for keeping a steady environment inside, which will help in developing spots. Also, it prevents solvent evaporation and keeps the entire process dust-free.
- Thin Layer Chromatography Mobile phase – The mobile phase is the one that moves and consists of a solvent mixture or a solvent. This phase should be particulate-free. The higher the quality of purity the development of spots is better.
- Thin Layer Chromatography Filter Paper – It has to be placed inside the It is moistened in the mobile phase.
Thin Layer Chromatography Experiment
The stationary phase applied to the plate is made to dry and stabilize.
- To apply sample spots, thin marks are made at the bottom of the plate with the help of a pencil.
- Apply sample solutions to the marked spots.
- Pour the mobile phase into the TLC chamber, and to maintain equal humidity, place a moistened filter paper in the mobile phase.
- Place the plate in the TLC chamber and close it with a lid. It is kept so that the sample faces the mobile phase.
- Immerse the plate for development. Remember to keep the sample spots well above the level of the mobile phase. Do not immerse it in the solvent.
- Wait till the development of spots. Once the spots are developed, take out the plates and dry The sample spots can be observed under a UV light chamber.
Thin Layer Chromatography Applications
- The qualitative testing of various medicines such as sedatives, local anesthetics, anticonvulsant tranquilizers, analgesics, antihistamines, steroids, and hypnotics is done by TLC.
- TLC is extremely useful in Biochemical analysis, such as separating or isolating biochemical metabolites from blood plasma, urine, body fluids, serum, etc.
- Thin-layer chromatography can identify natural products like essential or volatile oils, fixed oils, glycosides, waxes, alkaloids, etc.
- It is widely used in separating multicomponent pharmaceutical formulations.
- It is used to purify any sample, and a direct comparison is done between the sample and the authentic sample.
- It is used in the food industry to separate and identify colors, sweetening agents, and preservatives.
- It is used in the cosmetic industry.
Disadvantages of Thin Layer Chromatography:
Thin Layer Chromatography plates do not have a longer stationary
- Compared to other chromatographic techniques, the separation length is
- The results generated from TLC are difficult to
- Since TLC operates as an open system, some factors, such as humidity and temperature, can affect the outcome of the chromatogram.
- The detection limit is high, and therefore, if you want a lower detection limit, you cannot use TLC.
- It is only a qualitative analysis technique and not a quantitative one.
Ion Exchange Chromatography
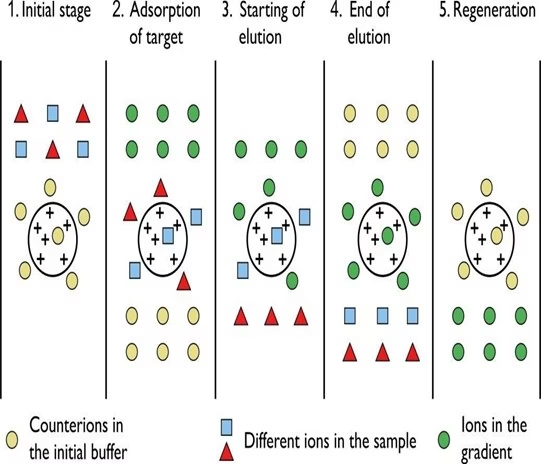
- Chromatography is the separation of a mixture of compounds into its components based on their relative interactions with an inert matrix.
- Ion exchange chromatography (or ion chromatography) is a process that allows the separation of ions and polar molecules based on their affinity to ion exchangers.
- The principle of separation is thus by reversible exchange of ions between the target ions present in the sample solution and the ions on ion exchangers.
- In this process, two types of exchangers, i.e., cationic and anionic exchangers, can be used.
- Cationic exchangers possess negatively charged groups, which will attract positively charged These exchangers are also called “Acidic ion exchange” materials because their negative charges result from the ionization of an acidic group.
- Anionic exchangers have positively charged groups that will attract negatively charged anions. These are also called “Basic ion exchange” materials.
- Ion exchange chromatography is most often performed as column chromatography. However, thin-layer chromatographic methods also work based on the principle of ion exchange.
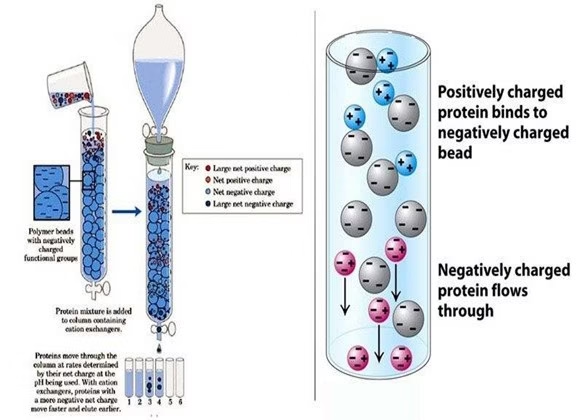
- Ion exchange chromatography is most often performed as column chromatography. However, thin-layer chromatographic methods also work based on the principle of ion exchange.
Principle of ion exchange chromatography
This form of chromatography relies on the attraction between the oppositely charged stationary phase, known as an ion exchanger, and analyte.
- The ion exchangers contain charged groups covalently linked to the surface of an insoluble matrix.
- The charged groups of the matrix can be positively or negatively
- When suspended in an aqueous solution, the charged groups of the matrix will be surrounded by ions of the opposite charge.
- In this “ion cloud”, ions can be reversibly exchanged without changing the matrix’s nature and properties.
Instrumentation
Typical IC instrumentation includes a pump, injector, column, suppressor, detector, and recorder or data system.
1. Pump
- The IC pump is considered one of the most important components in the system, providing a continuous flow of the eluent through the IC injector, column, and detector.
2. Injector
- Sample introduction can be accomplished in various ways.
- The simplest method is to use an injection valve.
- Liquid samples may be injected directly, and solid samples must only be dissolved in an appropriate solvent.
- Injectors should allow injecting the liquid sample within the range of 0.1 to 100 ml of volume with high reproducibility and under high pressure (up to 4000 psi).
3. Columns
- Depending on its ultimate use and area of application, the column material may be stainless steel, titanium, glass, or an inert plastic such as PEEK.
- The column can vary in diameter from about 2mm to 5 cm and in length from 3 cm to 50 cm, depending on whether it will be used for normal analytical purposes, microanalysis, high-speed analyses, or preparative work.
- The guard column is placed anterior to the separating column.
- This is a protective factor that prolongs the life and usefulness of the separation column.
- They are dependable columns designed to filter or remove particles that clog the separation column.
4. Suppressor
- The suppressor reduces the background conductivity of the chemicals used to elute samples from the ion exchange column, improving the conductivity measurement of the tested ions.
- IC suppressors are membrane-based devices that are designed to convert the ionic eluent to water as a means of enhancing the sensitivity.
5. Detectors
- An electrical conductivity detector is commonly used.
6. Data system
- A pre-programmed computing integrator may be sufficient for routine analysis without automation.
- A more intelligent device, such as a data station or minicomputer, is necessary for higher control levels.
Procedure of ion exchange chromatography
- Ion exchange separations are carried out mainly in columns packed with an ion exchanger.
- These ionic exchangers are commercially available. They are made up of styrene and divinyl benzene. Example. DEAE-cellulose is an anionic exchanger, and CM-cellulose is a cationic exchanger.
- The choice of the exchanger depends upon the particle’s charge to be separated. “An anionic exchanger” separates cations, and “A cationic exchanger” is used.
- First, the column is filled with an ion exchanger then the sample is applied, followed by the buffer. The tris-buffer, pyridine, acetate, citrate, and phosphate buffers are widely used.
- The particles with high affinity for ion exchanger will come down the column along with buffers.
- In the next step, the corresponding buffer separates the tightly bound particles.
- Then, these particles are analyzed spectroscopically.
Applications of ion exchange chromatography
- An important use of ion-exchange chromatography is in the routine analysis of amino acid mixtures.
- The 20 principal amino acids from blood serum or the hydrolysis of proteins are separated and used in clinical diagnosis.
- This is the most effective method for water purification. Complete deionization of water (or) a non-electrolyte solution is performed by exchanging solute cations for hydrogen ions and solute anions for hydroxyl ions. This is usually achieved by the method used to soften drinking water.
- In the analysis of products of hydrolysis of nucleic acids. In this way, information is gained about the structure of these molecules and how it relates to their biological function as carriers of hereditary information.
- Chelating resins are used to collect trace metals from seawater.
- To analyze lunar rocks and rare trace elements on Earth.
Gel Permeation Chromatography
Gel permeation chromatography is also called gel filtration or size exclusion
- In size exclusion chromatography, the stationary phase is a porous matrix comprising compounds like cross-linked polystyrene, cross-like dextrans, polyacrylamide gels, agarose gels, etc.
- The separation is based on the analyte molecular sizes since the gel behaves like a molecular sieve.
- This technique separates proteins, polysaccharides, enzymes, and synthetic polymers.
- As a technique, size exclusion chromatography was first developed in 1955 by Lathe and Ruthven.
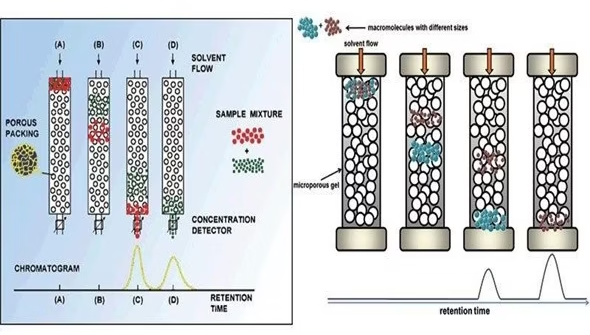
Principle of Gel Permeation Chromatography
- It is a technique in which components are separated based on the molecular weight or size difference.
- The stationary phase is a porous polymer matrix whose pores are filled with the solvent to be used as the mobile phase.
- The molecules in the sample are pumped through specialized columns containing microporous packing material (gel).
- The basis of the separation is that molecules above a certain size are excluded from the pores, while smaller molecules access the interior of the pores partly or
- The mobile phase’s flow will cause larger molecules to pass through the column unhindered without penetrating the gel matrix. In contrast, smaller molecules will be retarded according to their penetration of the gel.
Components
- Stationary Phase
- The Mobile Phase
- The Columns
- The Pump
- Detectors
A. Stationary phase
It comprises semi-permeable, porous polymer gel beads with a well-defined range of pore sizes.
It has the following properties:
- Chemically inert
- Mechanically stable
- With an ideal and homogeneous porous structure (wide pore size gives the low resolution).
- A uniform particle and pore
Examples of gel:
- Dextran (Sephadex) gel: An α 1-6-polymer of glucose natural gel
- Agarose gel: A 1,3 linked β-D-galactose and 1,4 linked 3,6-anhydro-α, L-galactose natural gel
- Acrylamide gel: A polymerized acrylamide, a synthetic gel
B. The Mobile Phase
It is composed of a liquid that dissolves the bio-molecules to make the mobile phase, permitting high detection response and wetting the packing surface.
C. Columns
Any of the following kinds may be used:
- Analytical column- 5–8mm diameters.
- Preparative columns-22–25mm
- Usual column lengths-25, 30, 50, and 60
- Narrow-bore columns- 2–3mm diameter have been introduced
D. Pumps
They are either syringes or reciprocating pumps with a high constant flow rate.
E. Detectors
The detectors may be concentration-sensitive, bulk property detectors, or refractive index (RI) detectors.
Steps in Gel Permeation Chromatography
It involves three major steps:
A. Preparation of column for gel filtration
It involves:
- Swelling of the gel
- Packing the column semi-permeable, porous polymer gel beads with a well-defined range of pore sizes.
- Washing: After packing, several column volumes of buffer solution are passed through the column to remove air bubbles and test the column homogeneity.
B. Loading the sample onto the column using a syringe
C. Eluting the sample and detection of components
Applications of Gel Permeation Chromatography
-
- Protein fractionation
- Purification
- Molecular weight determination.
- Separating sugar, proteins, peptides, rubbers, and others based on size.
- It can be used to determine the quaternary structure of purified protein.
Advantages of Gel Permeation Chromatography
- Short analysis time.
- Well-defined separation.
- Narrow bands and good sensitivity.
- There is no sample loss.
- A small amount of mobile phase is required.
- The flow rate can be set.
Limitations of Gel Permeation Chromatography
- The limited number of peaks can be resolved within the short time scale of the GPC run.
- Filtrations must be performed before using the instrument to prevent dust and other particulates from ruining the columns and interfering with the detectors.
- The molecular masses of most of the chains will be too close for the GPC separation to show anything more than broad peaks.
Affinity Chromatography
- Chromatography is an important biophysical technique that enables the separation, identification, and purification of the components of a mixture for qualitative and quantitative analysis.
- It is a separation technique in which a mobile phase carrying a mixture is caused to move in contact with a selectively absorbent stationary phase.
- Affinity chromatography is a type of liquid chromatography for the separation, purification or specific analysis of sample components.
- It utilizes the reversible biological interaction or molecular recognition called affinity, which refers to the attracting force exerted in different degrees between atoms, which causes them to remain in combination.
Example: Enzyme with an inhibitor, antigen with an antibody, etc.
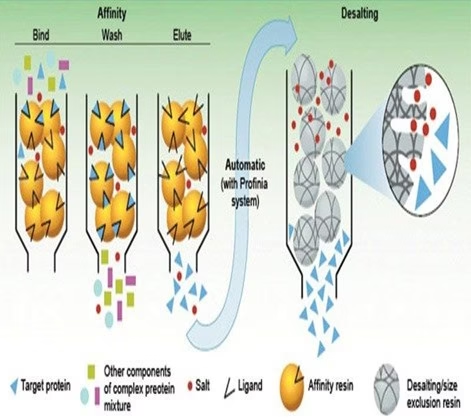
Principle of Affinity Chromatography
- The stationary phase consists of a support medium on which the substrate (ligand) is bound covalently so that the reactive groups essential for binding the target molecule are exposed.
- As the crude mixture of the substances is passed through the chromatography column, substances with binding sites for the immobilized substrate bind to the stationary phase. In contrast, all other substances are eluted in the column’s void volume.
- Once the other substances are eluted, the bound target molecules can be eluted by including a competing ligand in the mobile phase or changing the pH, ionic strength, or polarity conditions.
Components
1. Matrix
- The matrix is an inert support to which a ligand can be directly or indirectly coupled.
- For the matrix to be effective, it must have certain characteristics.
- Matrix should be chemically and physically inert.
- It must be insoluble in solvents and buffers employed in the process.
- It must be chemically and mechanically stable.
- It must be easily coupled to a ligand or spacer arm onto which the ligand can be attached.
- It must exhibit good flow properties and have a relatively large surface area for attachment.
- The most useful matrix materials are agarose and polyacrylamide.
2. Spacer arm
- It improves binding between ligand and target molecule by overcoming any effects of steric hindrance.
3. Ligand
- It refers to the molecule that binds reversibly to a specific target molecule.
- The ligand can be selected only after the isolated macromolecule’s nature is known.
- When a hormone receptor protein is to be purified by affinity chromatography, the hormone itself is an ideal candidate for the ligand.
- An antigen or hapten may be used as a ligand for antibody isolation.
- If an enzyme is to be purified, a substrate analog, inhibitor, cofactor, or effector may be used as an immobilized ligand.
Steps in Affinity Chromatography
- Affinity medium is equilibrated in binding buffer.
- The sample is applied under conditions that favor specific binding of the target molecule(s) to a complementary binding substance (the ligand). Target substances bind specifically, but reversibly, to the ligand, and unbound material washes through the column.
- Elution is performed specifically using a competitive ligand or non-specifically by changing the pH, ionic strength, or polarity. The target protein is collected in a purified, concentrated form.
- The affinity medium is re-equilibrated with a binding buffer.
These events can be summarized into the following three major steps:
1. Preparation of Column
- The column is loaded with solid support such as Sepharose, agarose, cellulose, etc.
- Ligand is selected according to the desired isolate.
- The spacer arm is attached between the ligand and the solid support.
2. Loading of Sample
- The solution containing a mixture of substances is poured into the elution column and allowed to run at a controlled rate.
3. Elution of Ligand-Molecule Complex
- The target substance is recovered by changing conditions to favor the elution of the bound molecule.
Applications of Affinity Chromatography
- Affinity chromatography is one of the most useful methods for separating and purifying specific products.
- It is a sample purification technique used primarily for biological molecules such as proteins.
Its major application includes:
- Separation of a mixture of components.
- Removal of impurities in the purification process.
- In enzyme assays.
- Detection of substrates.
- Investigation of binding sites of enzymes.
- In vitro antigen-antibody reactions.
- Detection of Single Nucleotide polymorphisms and mutations in nucleic acids.
Advantages of Affinity Chromatography
- High specificity.
- Target molecules can be obtained in a highly pure state.
- Single-step purification.
- The matrix can be reused rapidly.
- The matrix is a solid and can be easily washed and dried.
- Give purified products with high yield.
- Affinity chromatography can also remove specific contaminants, such as proteases.
Limitations of Affinity Chromatography
- Time consuming method.
- More amounts of solvents are required which may be expensive.
- Intense labor.
- Non-specific adsorption cannot be It can only be minimized.
- Limited availability and high cost of immobilized ligands.
- Proteins get denatured if the required pH is not adjusted.
Gas-Liquid Chromatography
- Gas-liquid chromatography (GLC) is a separation technique in which gas (usually inert gas, such as helium, or nonreactive gas, such as nitrogen) is used as a mobile phase (Figure 1) and liquid as a stationary phase.
- The basis of separation is the difference in the partition coefficient of volatilized compounds between liquid and gas phases when the desired compound is carried through the column by a carrier gas.
Principle
- GLC is based on the partitioning of compounds between stationary liquid and mobile gas phases. Due to its high sensitivity, reproducibility, and resolution speed, it is widely used for several qualitative and quantitative analyses.
- The gaseous compounds being analyzed interact with the walls of the column, which is coated with different kinds of stationary phases.
- This causes each compound to elute at different times (retention time), and then compare retention times. This makes GLC analytically very useful.
- As compound leaves the column, they pass through a detector linked to a chart recorder via an amplifier. The chart recorder records the peaks.
Applications
- Gas-liquid chromatography has many applications in separating and analyzing multi-component mixtures such as essential oils, hydrocarbons, and solvents. It is one of the primary analytical techniques used in forensic laboratories.
-
- GLC is combined with mass spectroscopy (GC/MS) for drug detection, fire investigation, environmental analysis, explosives investigation, and identification of unknown samples.
- GC/MS is also used in airport security to detect unwanted substances. Non-derivatized sugars and sugar alcohols are successfully analyzed by GC/MS using atmospheric pressure chemical ionization (APCI) in negative ion mode.
- Forensic scientists widely use GC to analyze body fluids for the presence of illegal substances, testing of fiber and blood from a crime scene, and detect residue from explosives.
- High-performance liquid chromatography, or HPLC, is an analytical technique that separates, identifies, or quantifies each component in a mixture.
- The mixture is separated using the basic principle of column chromatography and then identified and quantified by spectroscopy.
- In the 1960s, the column chromatography LC with its low-pressure suitable glass columns was further developed into the HPLC with its high-pressure adapted metal columns.
- Thus, HPLC is a highly improved form of column liquid chromatography. Instead of allowing a solvent to drip through a column under gravity, it is forced under high pressures of up to 400 atmospheres.
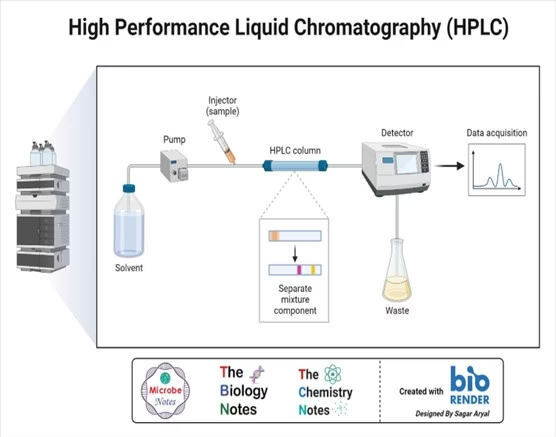
Principle of High-Performance Liquid Chromatography
- The purification occurs in a stationary and mobile phase separation column.
- The stationary phase is a granular material with very small porous particles in a separation column.
- The mobile phase, on the other hand, is a solvent or solvent mixture that is forced at high pressure through the separation column.
- Via a valve with a connected sample loop, e., a small tube or a capillary made of stainless steel, the sample is injected into the mobile phase flow from the pump to the separation column using a syringe.
- Subsequently, the individual components of the sample migrate through the column at different rates because they are retained to a varying degree by interactions with the stationary phase.
- After leaving the column, the individual substances are detected by a suitable detector and passed on as a signal to the HPLC software on the computer.
- At the end of this operation/run, a chromatogram in the HPLC software on the computer is obtained.
- The chromatogram allows the identification and quantification of the different substances.
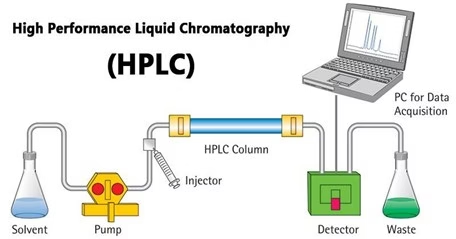
Instrumentation
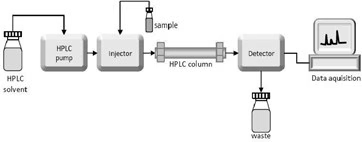
The Pump
- The development of HPLC led to the development of the pump system.
- The pump is positioned in the upper stream of the liquid chromatography system and generates a flow of eluent from the solvent reservoir into the system.
- High-pressure generation is a “standard” requirement of pumps, besides which it should also be able to provide a consistent pressure at any condition and a controllable and reproducible flow rate.
- Most pumps in current LC systems generate the flow by the back-and-forth motion of a motor-driven piston (reciprocating pumps). Because of this piston motion, it produces “pulses”.
Injector
- An injector is placed next to the pump.
- The simplest method uses a syringe, introducing the sample to the eluent flow.
- The most widely used injection method is based on sampling loops.
- An autosampler (auto-injector) system is also widely used, allowing repeated injections in a scheduled time.
Column
- The separation is performed inside the column.
- The recent columns are often prepared in a stainless steel housing instead of glass.
- The packing material generally used is silica or polymer gels compared to calcium. The eluent used for LC varies from acidic to basic solvents.
- Most column housing is made of stainless steel since stainless is tolerant towards many solvents.
Detector
- Separation of analytes inside the column, whereas a detector is used to observe the obtained separation.
- The composition of the eluent is consistent when no analyte is While the presence of analyte changes the composition of the eluent. What the detector does is to measure these differences.
- This difference is monitored as a form of an electronic signal. There are different types of detectors available.
Recorder
- The change in eluent detected by a detector is an electronic signal; thus, it is still not visible to our eyes.
- The pen (paper)-chart recorder was popular in the older days. Nowadays, a computer-based data processor (integrator) is more common.
- There are various types of data processors, from a simple system consisting of the in-built printer and word processor to those with software that is specifically designed for an LC system with not only data acquisition but features like peak-fitting, baseline correction, automatic concentration calculation, molecular weight determination, etc.
Degasser
The eluent used for LC analysis may contain gases such as oxygen that are non-visible to our eyes.
- When gas is present in the eluent, this is detected as noise and causes an unstable baseline.
- Degasser uses special polymer membrane tubing to remove gases.
- The numerous very small pores on the surface of the polymer tube allow the air to go through while preventing any liquid from going through the pore.
Column Heater
The LC separation is often largely influenced by the column temperature.
- To obtain repeatable results, it is important to keep consistent temperature conditions.
- Also for some analysis, such as sugar and organic acid, better resolutions can be obtained at elevated temperatures (50 to 80°C).
- Thus, columns are generally kept inside the column oven (column heater).
Types of High-Performance Liquid Chromatography
- Normal phase: – Column packing is polar (e.g., silica), and the mobile phase is non-polar. It is used for water-sensitive compounds, geometric isomers, cis-trans isomers, and chiral compounds.
- Reverse phase: – The column packing is non-polar (e.g., C18), and the mobile phase is water+ miscible solvent (e.g., methanol). It can be used for polar, non-polar, ionizable, and ionic samples.
- Ion exchange: – Column packing contains ionic groups, and the mobile phase is a buffer. It is used to separate anions and cations.
- Size exclusion: – Molecules diffuse into pores of a porous medium and are separated according to their relative size to the pore size. Large molecules elute first, and smaller molecules elute later.
Applications
The HPLC has developed into a universally applicable method so that it finds its use in almost all areas of chemistry, biochemistry, and pharmacy.
- Analysis of drugs
- Analysis of synthetic polymers
- Analysis of pollutants in environmental analytics
- Determination of drugs in biological matrices
- Isolation of valuable products
- Product purity and quality control of industrial products and fine chemicals
- Separation and purification of biopolymers such as enzymes or nucleic acids
- Water purification
- Pre-concentration of trace components
- Ligand-exchange chromatography
- Ion-exchange chromatography of proteins
- High-pH anion-exchange chromatography of carbohydrates and oligosaccharides
Advantages
- Speed
- Efficiency
- Accuracy
- Versatile and extremely precise when identifying and quantifying chemical
Limitations
- Cost: Despite its advantages, HPLC can be costly, requiring large quantities of expensive organics.
- Complexity
- HPLC does have low sensitivity for certain compounds, and some cannot be detected as they are irreversibly adsorbed.
- Volatile substances are better separated by gas chromatography.