Introduction
- A colorimeter is a device that measures the intensity of color or the absorbance of light by a solution.
- It is widely used in laboratories and industrial settings to determine the concentration of solutes in liquids based on their color.
- Colorimetric methods are typically applied when analyzing compounds that exhibit a distinct color, as their intensity is directly proportional to the concentration of the solute.
- Colorimeters are essential for pharmaceuticals, food production, environmental monitoring, and chemical manufacturing industries.
- The technique behind colorimetry is based on the understanding that light can be absorbed by colored substances, and the amount of absorbed light corresponds to the concentration of the substance.
- This principle is applied in various fields, from water quality testing to quality control in the food and beverage industry.

Principle of Colorimeter
The core principle of a colorimeter is based on the absorption of light by a solution containing a colored solute. The colorimeter typically measures two quantities: absorbance and transmittance.
- Absorbance: This refers to the amount of light absorbed by a sample at a particular wavelength. Absorbance is directly proportional to the concentration of the solute in the solution, and it can be measured using the Beer-Lambert Law.
- Transmittance: This is the ratio of the light that passes through the solution compared to the total light that was initially incident on the sample. Transmittance is inversely proportional to absorbance.
Beer-Lambert Law
The relationship between absorbance (A), concentration (C), and path length (L) is described by the Beer-Lambert law:
A=ε⋅C⋅L
Where:
- A is the absorbance,
- ε is the molar absorptivity (a constant that indicates how much light a substance absorbs at a particular wavelength),
- C is the concentration of the solute in the solution,
- L is the path length through the solution (usually the distance the light travels through the sample).
The principle suggests that as the concentration of the solute increases, the absorbance increases. This allows colorimeters to quantify a substance’s concentration by measuring light’s absorbance at a specific wavelength.
Parts of a Colorimeter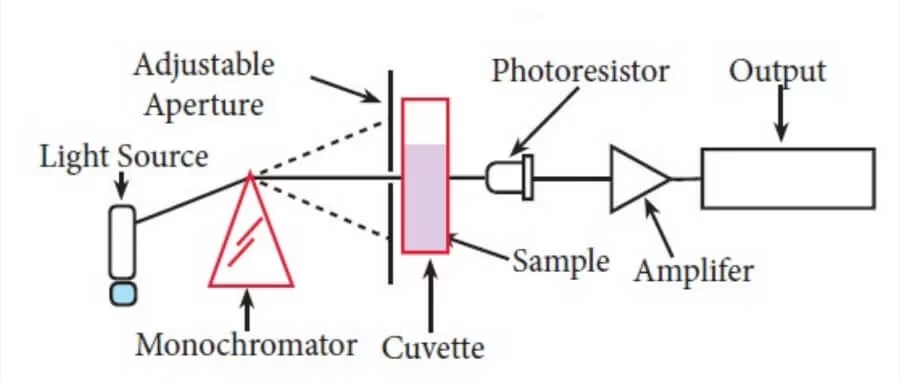
A colorimeter consists of several key components that work together to measure the color intensity of a sample. These parts include:
Light Source
The light source provides the necessary light that passes through the sample to measure its absorbance or transmittance. In most colorimeters, the light source is typically an LED (Light Emitting Diode) or a tungsten lamp.
- Purpose: To emit light of a specific wavelength or a narrow range of wavelengths that the sample absorbs.
- Types:
- Tungsten lamp: Used for visible light.
- LED: Common for specific wavelengths.
- Xenon lamp: Used in more advanced models for broader spectrum emission.
The choice of light source depends on the wavelength required for analysis, as different substances absorb light most effectively at different wavelengths.
Monochromator or Optical Filter
Once the light source emits light, the next step is to ensure that only the correct wavelength of light reaches the sample. This is where the monochromator or optical filter comes into play.
-
Monochromator:
- It is a device that isolates a single wavelength (or a narrow range) from a broad spectrum of light. This can be achieved using a prism, diffraction grating, or filters.
- More advanced colorimeters with monochromators can adjust the wavelength, allowing for flexibility in measurement, especially if the sample requires specific wavelengths.
-
Optical Filters:
- These are simpler, fixed devices that block certain wavelengths and only allow a specific wavelength or narrow band of wavelengths to pass through to the sample.
- Filters are more common in less expensive or portable models.
-
Purpose: To select the specific wavelength of light that the sample absorbs most efficiently.
Sample Holder (Cuvette)
The sample holder is where the sample solution is placed for measurement. Typically, this is a small container known as a cuvette.
-
Material: Cuvettes are often made of transparent glass, plastic, or quartz. The material must not absorb the wavelength of light used in the analysis, or it could interfere with the measurement.
-
Path Length: The cuvette’s path length is critical to absorbance measurements. The standard path length for most colorimeters is 1 cm. The absorbance is proportional to this path length, so cuvettes are standardized.
-
Shape: Cuvettes are typically rectangular or square with flat sides to ensure the light passes evenly through the sample.
-
Purpose: To hold the liquid sample in place, allowing the light to pass through it.
Photodetector (Sensor)
- After the light passes through the sample, the photodetector measures the amount of light that has been transmitted through the solution.
- The photodetector typically consists of a photodiode or photomultiplier tube (PMT), which converts the light into an electrical signal.
- The amount of light detected is inversely related to the concentration of the substance in the sample, as more concentrated solutions absorb more light.
- Function: Measures the transmitted light (or light absorbed by the sample).
- Types:
- Photodiodes: Convert light directly into electrical current and are commonly used in colorimeters.
- Photomultiplier Tubes (PMTs): Used for more sensitive measurements, converting low levels of light into readable electrical signals, though they are typically found in more advanced or laboratory-based instruments.
- Purpose: To detect the light that passes through the sample, allowing for calculating the concentration of the solute.
Readout Display or Processor
Once the detector has measured the light intensity, the colorimeter must process this information and convert it into meaningful data. The processed data is then displayed on a readout screen.
- Digital Display: Most modern colorimeters use a digital display that shows the absorbance or transmittance of the sample. Some advanced models may also display the concentration directly, based on pre-calibrated calibration curves.
- Analog Readout: In older or simpler models, colorimeters might have an analog dial that shows the intensity of absorbance or transmittance.
- Purpose: To present the results of the color measurement, often as a numeric value (absorbance, transmittance, or concentration).
Working Procedure
To use a colorimeter effectively, the following procedure is typically followed:
-
Calibration:
- Before using the colorimeter to analyze samples, it must be calibrated using a reference solution of known concentration.
- This ensures that the device is providing accurate measurements.
- Calibration usually involves setting the device to read zero for a blank sample (containing just the solvent) to account for any background light.
-
Choosing Wavelength:
- The wavelength of light to be used is chosen based on the absorption characteristics of the solute.
- For instance, if the solute absorbs light strongly at a specific wavelength, that wavelength will be selected for measurement.
- Some colorimeters may come with multiple filters to measure at different wavelengths.
-
Sample Preparation:
- The sample solution is prepared and placed into a cuvette.
- If necessary, the sample is diluted to ensure it falls within the range of the colorimeter’s measurement capabilities.
- The cuvette is then inserted into the colorimeter.
-
Measurement:
- The colorimeter shines light through the cuvette, and the photodetector measures how much light passes through the sample.
- Based on this, the absorbance or transmittance is calculated.
-
Analysis and Calculation:
- The colorimeter uses the Beer-Lambert Law or a calibration curve (a pre-determined graph of absorbance versus concentration) to calculate the solute concentration in the sample.
-
Results:
- The final result is displayed on the colorimeter’s readout as either absorbance, transmittance, or the concentration of the solute.
Applications
Colorimeters are used across various fields due to their simplicity, portability, and cost-effectiveness. Some common applications include:
-
Environmental Testing:
- Colorimeters are frequently used in environmental laboratories to analyze the concentration of pollutants in water, such as nitrates, phosphates, chlorine, and heavy metals.
- For example, they test drinking water quality and wastewater treatment.
-
Food and Beverage Industry:
- The food industry relies on colorimeters to ensure the consistency of product color.
- For example, they analyze the color of juices, sauces, oils, and other food products to maintain product uniformity and quality control.
-
Pharmaceutical Industry:
- In pharmaceutical laboratories, colorimeters analyze the concentration of active ingredients in solutions, such as in drug formulation and testing.
- This ensures that drugs meet their required concentration specifications.
-
Agriculture:
- In agriculture, colorimeters analyze soil samples and fertilizers to ensure proper nutrient levels.
- They can also be used to analyze the color of plants and fruits, which is often an indicator of their health or ripeness.
-
Clinical and Biochemical Research:
- In clinical laboratories, colorimeters analyze the concentration of substances in blood or urine samples, such as glucose, proteins, or cholesterol.
- They are also used in enzyme activity assays and other biochemical analyses.
Advantages
Colorimeters offer numerous benefits in scientific analysis:
-
Simplicity:
- Colorimeters are straightforward and require minimal training, making them accessible to technicians and researchers without advanced technical expertise.
-
Cost-Effective:
- Colorimeters are generally less expensive than more advanced instruments like spectrophotometers.
- This makes them a practical option for routine laboratory analysis and field testing.
-
Speed:
- The measurement process is fast, often providing results in seconds or minutes.
- This is particularly beneficial in industries where time-sensitive decisions must be made.
-
Portability:
- Modern handheld colorimeters are compact and portable, allowing for on-site analysis in various fields like environmental monitoring or field research.
-
Non-Destructive:
- The sample can be reused after measurement in many cases, as colorimetric analysis typically does not alter the sample significantly.
Disadvantages
Despite their advantages, colorimeters also have some limitations:
-
Limited Precision:
- Colorimeters may not be as precise as spectrophotometers, particularly when measuring very low or very high concentrations.
- This makes them unsuitable for ultra-sensitive applications.
-
Single Wavelength Limitation:
- Many colorimeters measure only at a single wavelength, which limits their ability to analyze samples that absorb light at multiple wavelengths.
- Spectrophotometers can measure absorbance over a broader range of wavelengths, making them more versatile.
-
Sample Interference:
- Other substances in the sample may interfere with the color measurement, leading to inaccurate results.
- For example, turbidity or particulate matter in the solution can scatter light, affecting the measurement.
-
Dependence on Calibration:
- Colorimeters require regular calibration to maintain accuracy.
- Any error in the calibration process can lead to incorrect readings, particularly when measuring complex samples.
Limitations
In addition to the disadvantages, colorimeters face some inherent limitations:
-
Limited Applicability:
- Colorimeters are only effective for analyzing colored solutions.
- They are unsuitable for colorless or faintly colored solutions, as there may not be enough light absorption to generate accurate results.
-
Temperature Sensitivity:
- The absorbance of light by a sample can be affected by temperature.
- Some colorimeters may not automatically compensate for temperature fluctuations, leading to potential inaccuracies if the sample temperature is not controlled.
-
Lower Sensitivity:
- Colorimeters may not be as sensitive as spectrophotometers, which makes them less suitable for measuring low concentrations of solutes.