Introduction
-
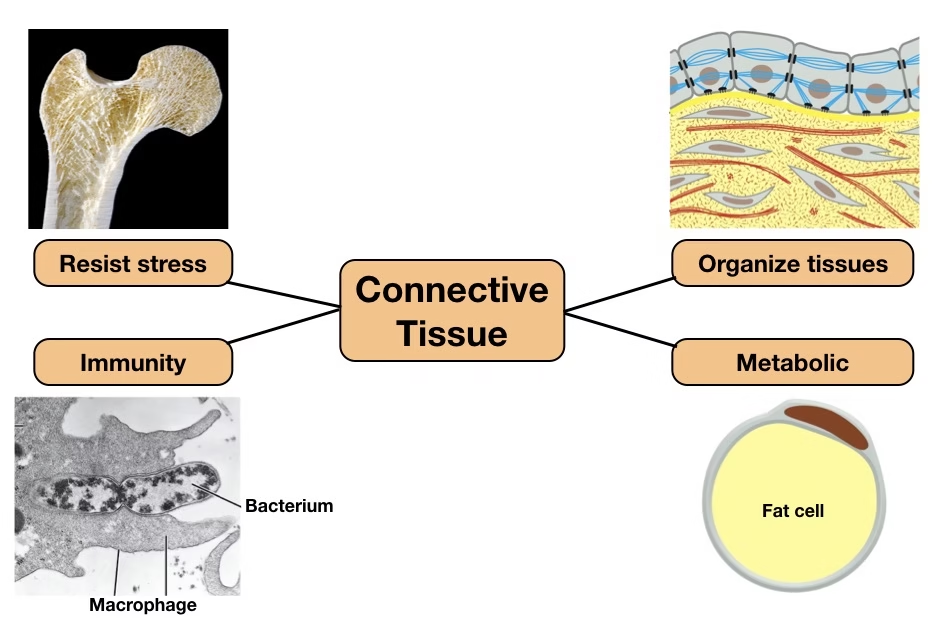
The connective tissue extracellular matrix is a mesoderm-derived tissue that provides structural support, strength, and metabolic integration to organs and tissues.
-
Biochemically, it is characterized by an abundant extracellular matrix (ECM) composed of collagen, elastin, proteoglycans, glycosaminoglycans, and adhesive glycoproteins.
-
The ECM is a dynamic biochemical system that regulates cell adhesion, migration, differentiation, and tissue remodeling.
-
Collagen, the most abundant protein in the body, forms the structural framework of bone, dentin, periodontal ligament, and oral mucosa.
-
Connective tissue metabolism depends on vitamins (especially vitamin C), trace elements (iron and copper), and specific enzymes, making it clinically significant.
-
Biochemical defects in connective tissue lead to disorders such as scurvy and osteogenesis imperfecta, which have direct dental and periodontal implications.
Classification of Connective Tissue
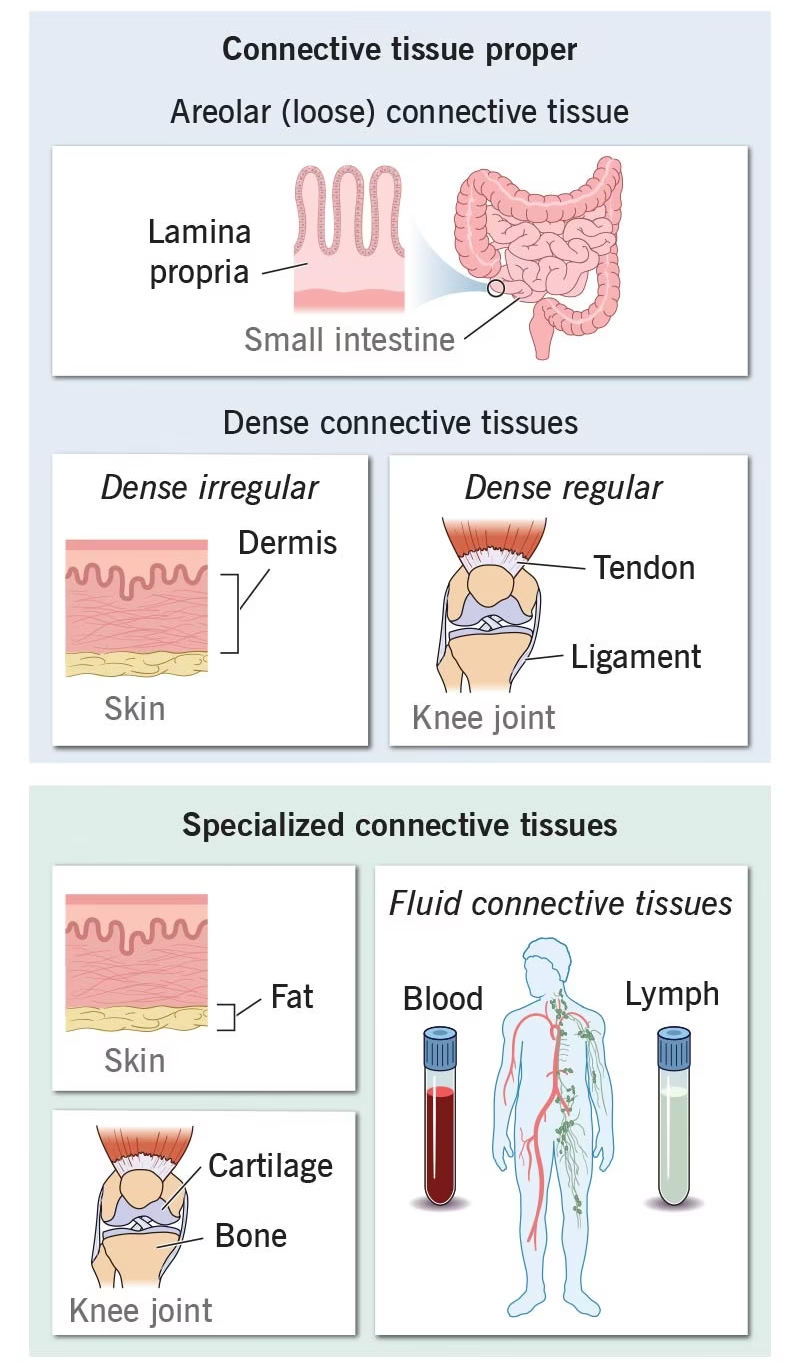
 I. Connective Tissue Proper
I. Connective Tissue Proper
A. Loose Connective Tissue
(Characterized by abundant ground substance and fewer fibers)
-
Areolar tissue
-
Widely distributed
-
Supports blood vessels and nerves
-
-
Adipose tissue
-
Specialized for fat storage
-
Endocrine and metabolic role
-
-
Reticular tissue
-
Network of reticular fibers (Type III collagen)
-
Supports lymphoid organs and pulp tissue

-
B. Dense Connective Tissue
(Characterized by abundant fibers and less ground substance)
-
Dense regular connective tissue
-
Parallel collagen fibers
-
Tendons and ligaments
-
-
Dense irregular connective tissue
-
Irregularly arranged collagen fibers
-
Dermis, capsules
-
-
Elastic connective tissue
-
Rich in elastic fibers
-
Blood vessels, ligaments
-
II. Specialized Connective Tissue
-
Cartilage
-
Hyaline
-
Elastic
-
Fibrocartilage
-
-
Bone
-
Compact bone
-
Spongy bone
-
-
Blood
-
Plasma (matrix)
-
Formed elements
-
III. Embryonic Connective Tissue
-
Mesenchyme
-
Undifferentiated connective tissue
-
Gives rise to all connective tissues
-
-
Mucous connective tissue
-
Wharton’s jelly
-
Rich in hyaluronic acid
-
Extracellular Matrix
- The extracellular matrix (ECM) is a complex, non-cellular biochemical network present between connective tissue cells.
- It provides structural support, mechanical strength, biochemical signaling, and metabolic regulation.
Composition of ECM
The ECM consists of two major components:
1. Fibrous Proteins
- Collagen fibers – tensile strength
- Elastic fibers – elasticity and recoil
- Reticular fibers – supportive framework
2. Ground Substance
- Glycosaminoglycans (GAGs)
- Proteoglycans
- Adhesive glycoproteins (fibronectin, laminin)
Biochemical Characteristics of ECM
- ECM is highly hydrated due to negatively charged GAGs.
- Acts as a molecular sieve, regulating diffusion of nutrients and metabolites.
- Serves as a reservoir for growth factors and cytokines.
- Continuously remodeled by matrix metalloproteinases (MMPs).
Functional Roles of ECM
- Maintains tissue architecture and integrity
- Regulates cell adhesion, migration, proliferation, and differentiation
- Provides mechanical resistance to compression and stretching
- Essential for wound healing and tissue repair
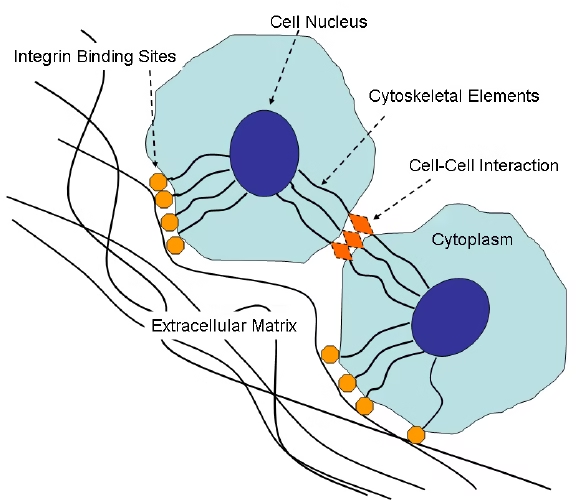
ECM–Cell Interaction
- Cells interact with ECM via integrins.
- ECM signals influence:
- Gene expression
- Cell survival
- Tissue specialization
Clinical Relevance
- Forms the matrix of dentin, pulp, periodontal ligament, and alveolar bone
- Essential for periodontal stability and implant integration
- Defective ECM metabolism results in:
- Bleeding gums
- Delayed wound healing
- Bone fragility
Fibers of Connective Tissue
Classification of Connective Tissue Fibers
1. Collagen Fibers
2. Elastic Fibers
3. Reticular Fibers
1. Collagen Fibers
General Features
-
Most abundant fibers in connective tissue
-
Composed mainly of collagen protein
-
Provide tensile strength and rigidity
-
Appear as thick, wavy, non-branching fibers
Molecular Structure
-
Basic unit: Tropocollagen
-
Triple-helical structure with three α-chains
-
Repeating amino acid sequence:
Gly–X–Y-
X = Proline
-
Y = Hydroxyproline / Hydroxylysine
-
Glycine at every third position allows tight helix formation.
Types of Collagen (High-Yield)
| Type | Major Location | Dental Relevance |
|---|---|---|
| Type I | Bone, dentin, tendon | Dentin & alveolar bone |
| Type II | Cartilage | TMJ |
| Type III | Blood vessels, pulp | Pulp tissue |
| Type IV | Basement membrane | Oral epithelium |
| Type V | Placenta, cornea | Tooth development |
Collagen Biosynthesis (Intracellular + Extracellular)
Intracellular (RER & Golgi)
-
Synthesis of preprocollagen
-
Removal of signal peptide → procollagen
-
Hydroxylation of proline & lysine
-
Requires:
-
Vitamin C
-
Iron (Fe²⁺)
-
Oxygen
-
α-ketoglutarate
-
-
-
Glycosylation of hydroxylysine
-
Triple helix formation
Extracellular
-
Secretion of procollagen
-
Cleavage → tropocollagen
-
Cross-linking by lysyl oxidase
-
Copper-dependent enzyme
-
Clinical Correlation
-
Vitamin C deficiency → Scurvy
-
Defective hydroxylation
-
Weak collagen
-
Bleeding gums, loose teeth
-
-
Copper deficiency
-
Poor cross-linking
-
Fragile connective tissue
-
-
Osteogenesis imperfecta → Type I collagen defect → fragile teeth
2. Elastic Fibers
Composition
-
Core protein: Elastin
-
Microfibrillar component: Fibrillin
Biochemical Features
-
Rich in desmosine and isodesmosine cross-links
-
Highly elastic and extensible
-
Resistant to proteolytic enzymes
Function
-
Allows tissues to stretch and recoil
-
Maintains elasticity of:
-
Blood vessels
-
Lungs
-
Periodontal ligament
-
Clinical Correlation
-
Defective fibrillin → Marfan syndrome (elastic fiber abnormality)
3. Reticular Fibers
Composition
-
Composed of Type III collagen
-
Thin, branching fibers
-
Rich in carbohydrates
Characteristics
-
Form a supportive meshwork (scaffold)
-
Argyrophilic (silver-staining)
Distribution
-
Lymphoid organs
-
Liver and spleen
-
Pulp tissue
-
Bone marrow
Comparation
| Feature | Collagen | Elastic | Reticular |
|---|---|---|---|
| Protein | Collagen | Elastin | Type III collagen |
| Function | Strength | Elasticity | Support |
| Thickness | Thick | Thin | Very thin |
| Special stain | Eosin | Orcein | Silver stain |
Ground Substance
-
Ground substance is an amorphous, gel-like component of the extracellular matrix that occupies the space between cells and fibers.
-
It is biochemically active and plays a key role in diffusion, hydration, and mechanical support.
Composition of Ground Substance
Ground substance is composed mainly of:
-
Glycosaminoglycans (GAGs)
-
Proteoglycans
-
Adhesive glycoproteins
Glycosaminoglycans (GAGs)
Characteristics
-
Long, unbranched polysaccharides
-
Repeating disaccharide units
-
Highly negatively charged
-
Bind Na⁺ and water → hydration
Types
| GAG | Location | Importance |
|---|---|---|
| Hyaluronic acid | ECM, synovial fluid | Lubrication |
| Chondroitin sulfate | Cartilage, bone | Shock absorption |
| Dermatan sulfate | Skin, blood vessels | Structural integrity |
| Heparan sulfate | Basement membrane | Filtration |
Proteoglycans
Structure
-
Core protein + multiple GAG chains
-
Form large aggregates with hyaluronic acid
Functions
-
Provide resistance to compression
-
Regulate diffusion of molecules
-
Bind growth factors
Adhesive Glycoproteins
Important Examples
-
Fibronectin
-
Cell adhesion
-
Wound healing
-
-
Laminin
-
Basement membrane integrity
-
Epithelial attachment
-
Basement Membrane
-
The basement membrane (BM) is a specialized extracellular matrix (ECM) that lies between epithelial cells and underlying connective tissue.
-
It provides structural support, selective permeability, and biochemical signaling.
Structural Organization
The basement membrane has two layers:
-
Basal lamina (epithelial origin)
-
Lamina lucida
-
Lamina densa
-
-
Reticular lamina (connective tissue origin)
Molecular Composition
-
Type IV collagen – forms a sheet-like network (not fibrillar)
-
Laminin – cell adhesion and organization
-
Nidogen (entactin) – links laminin to collagen IV
-
Heparan sulfate proteoglycan – negative charge and filtration
Biochemical Characteristics
-
Rich in non-fibrillar collagen
-
Highly organized protein–carbohydrate matrix
-
Negatively charged → regulates molecular diffusion
-
Acts as a reservoir for growth factors
Functions
-
Anchors epithelium to connective tissue
-
Maintains tissue polarity
-
Regulates cell differentiation and migration
-
Acts as a selective permeability barrier
-
Essential for wound healing and regeneration
Cell–Basement Membrane Interaction
-
Cells attach to BM via integrins
-
Integrin signaling influences:
-
Gene expression
-
Cell survival
-
Tissue specialization
-
Clinical Significance
-
Supports oral epithelium
-
Maintains integrity of gingiva and oral mucosa
-
Essential for periodontal attachment
-
Important in wound healing after extraction and surgery
Role of Connective Tissue
-
Structural support: Forms the framework of organs and tissues; provides strength to bone, dentin, periodontal ligament, and oral mucosa.
-
Mechanical functions:
-
Collagen → tensile strength
-
Elastic fibers → elasticity and recoil
-
Ground substance → resistance to compression
-
-
Metabolic exchange: Facilitates diffusion of nutrients, gases, metabolites, and signaling molecules between blood and cells.
-
Cell–matrix signaling: Regulates cell adhesion, migration, proliferation, and differentiation via ECM–integrin interactions.
-
Tissue remodeling: Undergoes continuous turnover mediated by enzymes (e.g., MMPs); essential for growth, repair, and adaptation.
-
Wound healing: Provides scaffold for cell migration, angiogenesis, and re-epithelialization after injury or dental procedures.
-
Protective role: Acts as a barrier and shock absorber, especially in joints, pulp, and periodontal tissues.
-
Dental relevance: Critical for periodontal stability, orthodontic tooth movement, implant integration, and post-extraction healing.
Metabolic Disorders of Connective Tissue
Metabolic disorders of connective tissue arise due to defects in collagen synthesis, cross-linking, proteoglycan metabolism, or degradation of amino acids, leading to structural and functional abnormalities.
1. Scurvy
-
Cause: Vitamin C deficiency
-
Biochemical defect:
-
Impaired hydroxylation of proline and lysine
-
Defective collagen triple-helix stability
-
-
Clinical features:
-
Bleeding gums
-
Loose teeth
-
Delayed wound healing
-
-
Dental significance:
-
Periodontal weakness and gingival bleeding
-
2. Osteogenesis Imperfecta
-
Cause: Genetic defect in Type I collagen
-
Biochemical defect:
-
Abnormal collagen synthesis or structure
-
-
Clinical features:
-
Brittle bones
-
Blue sclera
-
Hearing loss
-
-
Dental significance:
-
Fragile teeth
-
Dentinogenesis imperfecta
-
3. Ehlers–Danlos Syndrome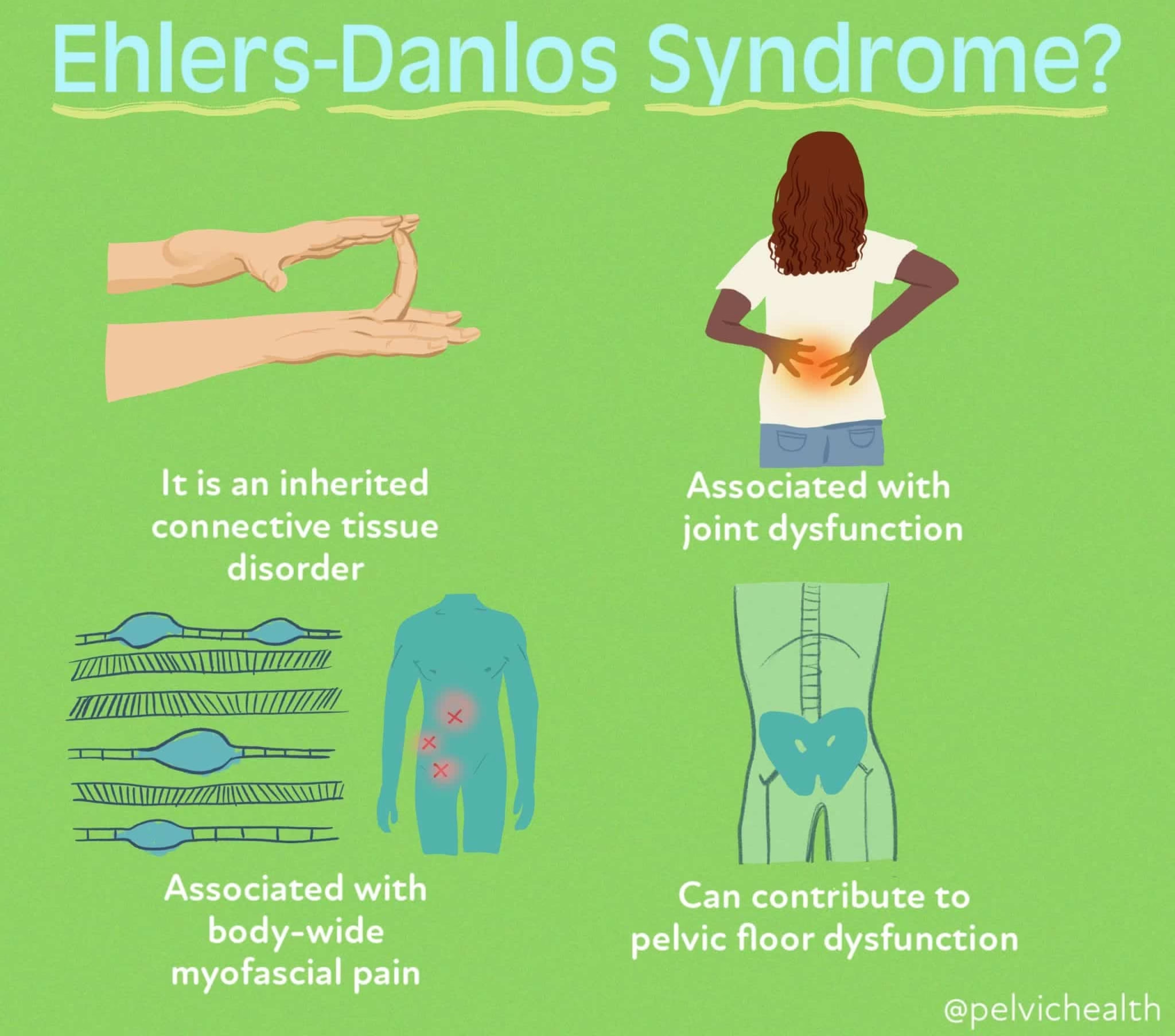
-
Cause: Defective collagen cross-linking or synthesis
-
Biochemical defect:
-
Abnormal collagen fiber organization
-
-
Clinical features:
-
Hyperextensible skin
-
Joint hypermobility
-
-
Dental significance:
-
Periodontal fragility
-
Increased risk of tissue injury during procedures
-
4. Alkaptonuria
-
Cause: Homogentisic acid oxidase deficiency
-
Biochemical defect:
-
Accumulation of homogentisic acid
-
-
Clinical features:
-
Ochronosis (dark pigmentation of connective tissue)
-
Arthritis
-
-
Dental significance:
-
Pigmentation of oral tissues in advanced cases
-
5. Menkes Disease
-
Cause: Copper metabolism defect
-
Biochemical defect:
-
Reduced activity of lysyl oxidase
-
Poor collagen cross-linking
-
-
Clinical features:
-
Fragile connective tissue
-
Skeletal abnormalities
-
MCQs
1. Connective tissue is primarily derived from:
A. Ectoderm
B. Endoderm
C. Mesoderm
D. Neuroectoderm
2. The most abundant protein in the human body is:
A. Elastin
B. Actin
C. Collagen
D. Keratin
3. Basic repeating amino acid sequence of collagen is:
A. Ala–Gly–Pro
B. Gly–X–Y
C. Pro–Pro–Gly
D. Lys–Gly–Ala
4. Which amino acid occurs at every third position in collagen?
A. Proline
B. Lysine
C. Glycine
D. Hydroxylysine
5. Hydroxylation of proline and lysine during collagen synthesis requires:
A. Vitamin A
B. Vitamin D
C. Vitamin C
D. Vitamin K
6. Enzyme responsible for collagen cross-linking is:
A. Prolyl hydroxylase
B. Lysyl hydroxylase
C. Lysyl oxidase
D. Collagenase
7. Copper deficiency mainly affects:
A. Collagen hydroxylation
B. Collagen glycosylation
C. Collagen cross-linking
D. Triple helix formation
8. Type I collagen is predominantly found in:
A. Cartilage
B. Basement membrane
C. Bone and dentin
D. Blood vessels
9. Type II collagen is a major component of:
A. Bone
B. Cartilage
C. Skin
D. Tendon
10. Reticular fibers are composed mainly of:
A. Elastin
B. Type I collagen
C. Type III collagen
D. Type IV collagen
11. Reticular fibers are best demonstrated by:
A. PAS stain
B. Orcein stain
C. Silver stain
D. H&E stain
12. Elastic fibers owe their elasticity to:
A. Collagen
B. Fibrillin
C. Elastin
D. Laminin
13. Unique cross-linking amino acids of elastin are:
A. Hydroxyproline
B. Desmosine and isodesmosine
C. Hydroxylysine
D. Cysteine
14. Major function of elastic fibers is:
A. Tensile strength
B. Shock absorption
C. Elastic recoil
D. Lubrication
15. Extracellular matrix (ECM) mainly consists of:
A. Cells only
B. Fibers and ground substance
C. Blood vessels
D. Nerve fibers
16. Ground substance is best described as:
A. Solid matrix
B. Fibrous network
C. Gel-like amorphous substance
D. Crystalline structure
17. Glycosaminoglycans are characterized by:
A. Neutral charge
B. Positive charge
C. Negative charge
D. Hydrophobic nature
18. Most abundant GAG in ECM is:
A. Chondroitin sulfate
B. Dermatan sulfate
C. Hyaluronic acid
D. Heparan sulfate
19. Hyaluronic acid differs from other GAGs because it:
A. Is sulfated
B. Is protein-bound
C. Is not sulfated
D. Is intracellular
20. Proteoglycans consist of:
A. Only proteins
B. Only carbohydrates
C. Core protein + GAG chains
D. Lipoproteins
21. Proteoglycans primarily provide:
A. Tensile strength
B. Elastic recoil
C. Resistance to compression
D. Oxygen transport
22. Adhesive glycoprotein involved in wound healing is:
A. Laminin
B. Fibronectin
C. Elastin
D. Keratan sulfate
23. Basement membrane collagen is:
A. Type I
B. Type II
C. Type III
D. Type IV
24. Major adhesive protein of basement membrane is:
A. Fibronectin
B. Laminin
C. Elastin
D. Aggrecan
25. Heparan sulfate in basement membrane mainly provides:
A. Tensile strength
B. Elasticity
C. Charge selectivity
D. Pigmentation
26. Cells attach to ECM via:
A. Cadherins
B. Integrins
C. Selectins
D. Immunoglobulins
27. Scurvy is caused due to deficiency of:
A. Vitamin A
B. Vitamin B12
C. Vitamin C
D. Vitamin D
28. Primary biochemical defect in scurvy is:
A. Reduced collagen synthesis
B. Defective hydroxylation
C. Poor glycosylation
D. Increased degradation
29. A genetic defect of Type I collagen causes:
A. Scurvy
B. Alkaptonuria
C. Osteogenesis imperfecta
D. Marfan syndrome
30. Dental manifestation of osteogenesis imperfecta includes:
A. Gingival hyperplasia
B. Dentinogenesis imperfecta
C. Dental caries
D. Enamel hypoplasia
31. Ehlers–Danlos syndrome is characterized by:
A. Rigid joints
B. Hyperextensible skin
C. Brittle bones
D. Ochronosis
32. Enzyme deficient in alkaptonuria is:
A. Tyrosinase
B. Homogentisic acid oxidase
C. Phenylalanine hydroxylase
D. Lysyl oxidase
33. Accumulation of homogentisic acid causes:
A. Jaundice
B. Ochronosis
C. Scurvy
D. Rickets
34. Major function of ground substance hydration is:
A. Rigidity
B. Lubrication and diffusion
C. Pigmentation
D. Electrical conduction
35. Which connective tissue fiber provides tensile strength?
A. Elastic
B. Reticular
C. Collagen
D. Oxytalan
36. Reticular fibers form supportive framework in:
A. Tendons
B. Lymphoid organs
C. Cartilage
D. Blood vessels
37. ECM is best described as:
A. Inert structure
B. Metabolically inactive
C. Biochemically dynamic
D. Crystalline matrix
38. Matrix metalloproteinases (MMPs) are involved in:
A. Collagen synthesis
B. ECM degradation
C. ECM glycosylation
D. Basement membrane formation
39. Periodontal ligament elasticity is mainly due to:
A. Collagen fibers
B. Reticular fibers
C. Elastic fibers
D. Basement membrane
40. Ground substance mainly resists:
A. Stretching
B. Compression
C. Tearing
D. Bending
41. Basement membrane is located between:
A. Muscle and bone
B. Epithelium and connective tissue
C. Bone and cartilage
D. Nerve and muscle
42. Laminin mainly functions in:
A. Elastic recoil
B. Cell adhesion
C. Compression resistance
D. Pigmentation
43. Glycine is essential in collagen because it:
A. Forms cross-links
B. Allows tight helix packing
C. Binds calcium
D. Provides elasticity
44. Which GAG is abundant in cartilage?
A. Hyaluronic acid
B. Chondroitin sulfate
C. Heparin
D. Keratan sulfate only
45. Which connective tissue component binds growth factors?
A. Collagen
B. Proteoglycans
C. Elastin
D. Actin
46. Collagen biosynthesis begins in:
A. Golgi apparatus
B. Cytosol
C. Rough endoplasmic reticulum
D. Lysosome
47. Triple helix collagen molecule is called:
A. Procollagen
B. Tropocollagen
C. Elastin
D. Aggrecan
48. Oral mucosal integrity is maintained mainly by:
A. Elastic fibers
B. Basement membrane
C. Adipose tissue
D. Blood
49. Which component gives ECM its gel-like nature?
A. Collagen
B. Elastin
C. Ground substance
D. Reticular fibers
50. Connective tissue plays a role in all EXCEPT:
A. Structural support
B. Cell signaling
C. Hormone synthesis
D. Wound healing
Answer Key
-
C
-
C
-
B
-
C
-
C
-
C
-
C
-
C
-
B
-
C
-
C
-
C
-
B
-
C
-
B
-
C
-
C
-
C
-
C
-
C
-
C
-
B
-
D
-
B
-
C
-
B
-
C
-
B
-
C
-
B
-
B
-
B
-
B
-
B
-
C
-
B
-
C
-
B
-
C
-
B
-
B
-
B
-
B
-
B
-
B
-
C
-
B
-
B
-
C
-
C