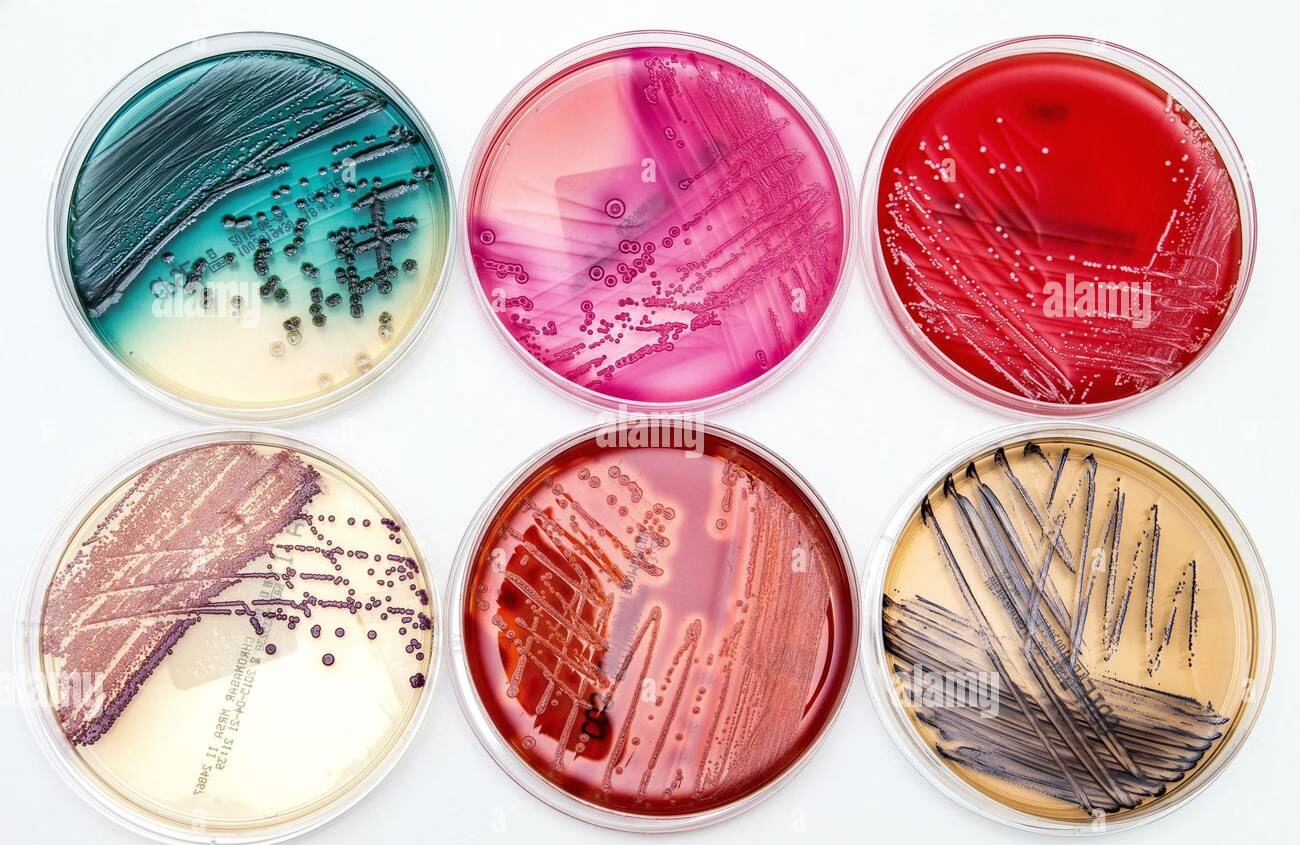
Introduction
- Culture media are essential for supporting the growth of microorganisms in various laboratory applications.
- Understanding the roles of each component, from nutrient sources to solidifying agents, is crucial for microbiologists to prepare effective media tailored to their specific needs.
- Various types of culture media and their specific uses are crucial for microbiologists in research, clinical diagnostics, and industrial applications.
- Each media type serves a specific purpose: to support general growth, enrich fastidious organisms, selectively isolate specific bacteria, or facilitate differentiation based on biochemical activity.
- By selecting the appropriate media, microbiologists can obtain accurate results and better understand the microorganisms they study.
Basic Components of Culture Media
1. Water
Water is the main component of any culture medium.
It provides:
-
A solvent for nutrients
-
A medium for biochemical reactions
-
Proper hydration for microbial cells
Distilled or deionized water is used to avoid impurities.
2. Carbon Source
Microorganisms need carbon for energy and cell structure.
Common carbon sources in media include:
-
Glucose (most widely used)
-
Lactose, sucrose
-
Starch
Glucose supports rapid growth and fermentation reactions.
3. Nitrogen Source
Nitrogen is essential for building:
-
Proteins
-
Nucleic acids
-
Enzymes
Common nitrogen sources:
-
Peptones (partially digested proteins)
-
Tryptone
-
Yeast extract
These provide amino acids, peptides, and growth factors.
4. Mineral Salts
Microbes require small amounts of minerals for enzyme activity and metabolism.
Examples include:
-
Sodium chloride (NaCl) – maintains osmotic balance
-
Magnesium sulfate (MgSO₄) – enzyme cofactor
-
Potassium phosphate – provides buffering and phosphorus
Mineral salts support overall cell function.
5. Vitamins & Growth Factors
Some organisms, especially fastidious ones, require additional nutrients such as:
-
Vitamins (B-complex)
-
Amino acids
-
Purines and pyrimidines
-
Blood, serum, or egg (in enriched media)
These enhance growth and survival of nutritionally demanding microbes.
6. Buffers
Buffers maintain a stable pH during microbial growth.
Common buffers:
-
Phosphates
-
Peptone water
A stable pH prevents inhibition of microbial growth and ensures accurate biochemical reactions.
7. Selective Agents (Inhibitors)
These components help suppress unwanted organisms while allowing the target microbes to grow.
Examples:
-
Bile salts – inhibit Gram-positive bacteria
-
Antibiotics – used in selective fungal or mycobacterial media
-
Dyes (e.g., crystal violet) – inhibit specific bacterial groups
-
High salt concentration – selects Staphylococci (e.g., MSA)
These agents help isolate specific groups of bacteria.
8. Indicators
Indicators produce color changes based on pH or biochemical activity, helping differentiate organisms.
Common indicators:
-
Phenol red (changes from yellow to red)
-
Neutral red (used in MacConkey agar)
-
Bromothymol blue
Indicators are essential in differential media.
9. Agar (Solidifying Agent)
Agar is a jelly-like substance obtained from seaweed and used to solidify media.
Properties:
-
Melts at 90–100°C
-
Solidifies at 40–45°C
-
Not digested by most bacteria
-
Provides a solid surface for colony formation
Agar makes it possible to isolate pure colonies.
Summary Table of Basic Components
| Component | Purpose | Examples |
|---|---|---|
| Water | Solvent & reaction medium | Distilled water |
| Carbon source | Energy & biomass | Glucose, sucrose |
| Nitrogen source | Protein & nucleic acid synthesis | Peptone, yeast extract |
| Minerals | Enzyme activation, osmosis | NaCl, MgSO₄, phosphates |
| Vitamins/Growth factors | For fastidious organisms | Blood, serum, B-vitamins |
| Buffers | Maintain pH | Phosphate buffer |
| Selective agents | Inhibit unwanted microbes | Bile salts, antibiotics |
| Indicators | Show color change | Phenol red, neutral red |
| Agar | Solidifies media | 1.5–2% agar |
Types of Culture Media
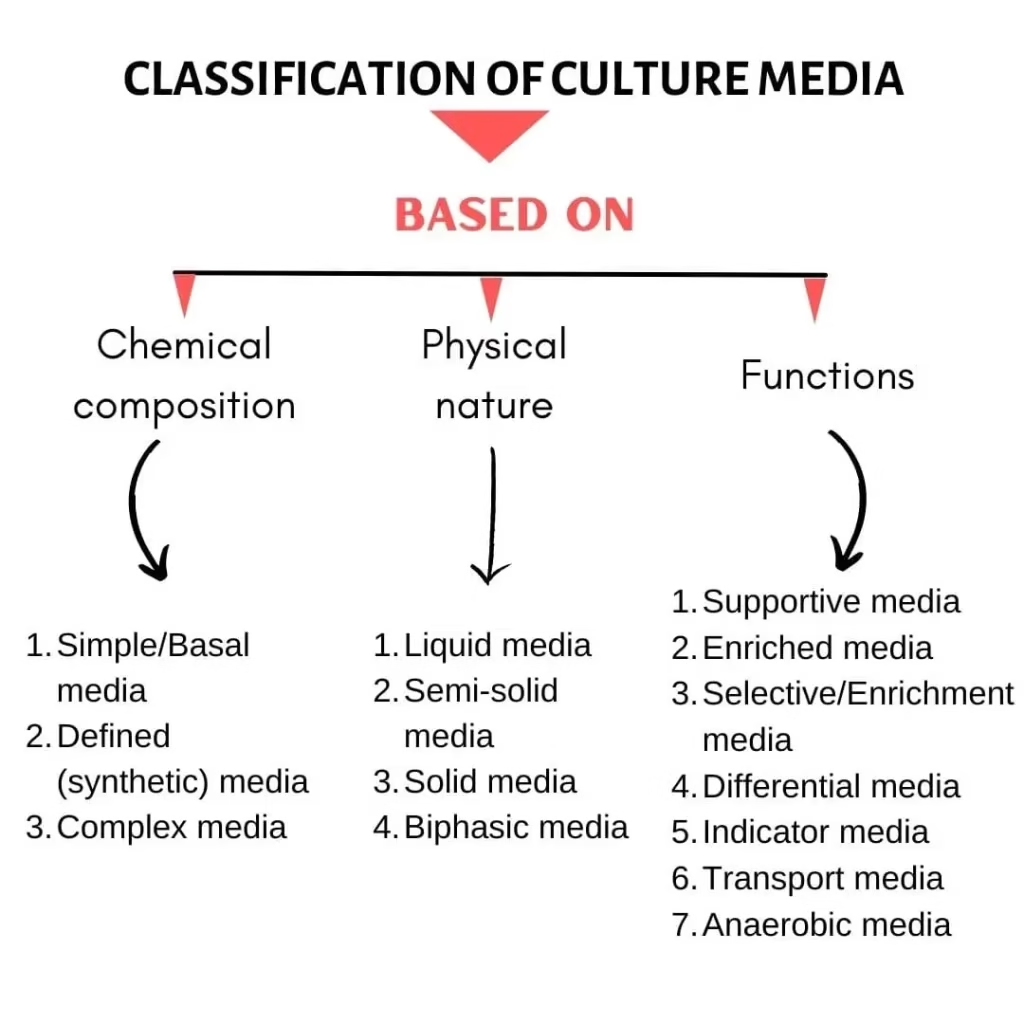 1. Based on Physical Consistency
1. Based on Physical Consistency
A. Liquid Media (Broth)
-
Do not contain agar
-
Used for growing large numbers of bacteria quickly
-
Useful for biochemical tests and enrichment
Examples: Nutrient broth, Tryptic soy broth (TSB)

B. Solid Media
-
Contain 1.5–2% agar
-
Provide a solid surface for colony formation
-
Helpful in isolating pure cultures
Examples: Nutrient agar, Blood agar, MacConkey agar
C. Semi-solid Media
-
Contain 0.3–0.5% agar
-
Soft consistency
-
Used for motility testing and microaerophilic cultures
Examples: SIM medium, Motility agar
2. Based on Chemical Composition
A. Simple (Basal) Media
Support the growth of non-fastidious organisms.
Examples: Nutrient broth, Nutrient agar
B. Complex Media
Exact chemical composition is not known.
Contain peptones, meat extract, yeast extract, etc.
Examples: MacConkey agar, Blood agar
C. Synthetic (Defined) Media
Exact chemical formula for all components is known.
Used in research and special studies.
Examples: Minimal media for E. coli
D. Enriched Media
Basal media enriched with blood, serum, or egg to support fastidious organisms.
Examples:
-
Blood agar
-
Chocolate agar
-
Loeffler’s serum medium
3. Based on Function or Purpose
A. Enrichment Media
Liquid media that enhance the growth of desired pathogens while inhibiting others.
Used for samples with low numbers of target organisms.
Examples:
-
Selenite F broth (for Salmonella)
-
Alkaline peptone water (for Vibrio)
B. Selective Media
Allow the growth of specific microbes while suppressing others using inhibitors such as dyes, antibiotics, or bile salts.
Examples:
-
MacConkey agar → inhibits Gram-positive bacteria
-
Mannitol Salt Agar (MSA) → high salt selects Staphylococcus
-
TCBS agar → selective for Vibrio species
-
Cetrimide agar → selects Pseudomonas aeruginosa
C. Differential (Indicator) Media
Show visible changes (usually color changes) that help differentiate organisms based on biochemical reactions.
Examples:
-
MacConkey agar → lactose fermenters (pink) vs. non-fermenters (colorless)
-
Blood agar → hemolysis patterns (α, β, γ)
-
TCBS agar → sucrose fermenters (yellow) vs. non-fermenters (green)
D. Transport Media
Used to preserve microorganisms during transport from collection site to the laboratory. Prevent multiplication but maintain viability.
Examples:
-
Stuart’s transport medium
-
Amies medium
-
Cary-Blair medium
E. Anaerobic Media
Provide conditions suitable for the growth of anaerobic bacteria. Often contain reducing agents like thioglycolate.
Examples:
-
Robertson Cooked Meat (RCM) medium
-
Thioglycolate broth
-
Anaerobic blood agar
F. Assay Media
Used in antibiotic sensitivity testing, vitamin assays, etc.
Examples:
-
Mueller-Hinton agar (for antibiotic susceptibility tests)
-
Antibiotic assay media (used in pharmaceutical tests)
G. Storage / Preservation Media
Used for long-term storage of microorganisms.
Examples:
-
Freeze-drying (lyophilization) medium
-
Skim milk medium
-
Glycerol broth for −80°C storage
4. Special Media
These include media with unique or specialized functions:
A. Chromogenic Media
Contain chromogenic substrates that release colored compounds when specific enzymes act.
Used for rapid identification.
Example: Chromogenic UTI agar.
B. Indicator Media
Contain pH or chemical indicators.
Example: TSI (Triple Sugar Iron) medium.
C. Differential Selective Media
Have both selective agents and indicators.
Example: MacConkey agar (selective + differential).
Summary Table: Types of Culture Media
| Classification Basis | Types | Examples |
|---|---|---|
| Consistency | Liquid, Solid, Semi-solid | Broth, Nutrient agar, SIM |
| Composition | Simple, Complex, Synthetic, Enriched | NA, Blood agar, Minimal media |
| Function | Enrichment, Selective, Differential, Transport, Anaerobic | Selenite F, MacConkey, Stuart’s |
| Special Media | Chromogenic, Assay, Preservation | MH agar, Chromogenic UTI |
Methods of Culture
1. Streak Plate Method
What it is:
A method to separate bacteria on the surface of a solid agar plate.
How it is done:
-
Take a sterile loop.
-
Dip it in the sample.
-
Spread it on the agar plate in 3–4 different directions (called “quadrants”).
-
Each step reduces the number of bacteria.
What you get:
After incubation, you see single, well-separated colonies.
Why this method is important:
-
It helps in getting a pure culture.
-
Very useful for identifying bacteria based on colony shape, color, and size.
2. Spread Plate Method
What it is:
A method to spread bacteria evenly across the surface of agar.
How it is done:
-
Put a small measured amount (0.1 mL) of diluted sample on the agar.
-
Use a glass spreader to spread it like butter on bread.
What you get:
Bacteria grow as scattered colonies all over the plate.
Where it is used:
-
To count the number of bacteria (CFU).
-
In antibiotic testing where even growth is needed.
3. Pour Plate Method
What it is:
A method where bacteria are mixed with liquid agar and then poured into Petri plates.
How it is done:
-
Take a diluted sample.
-
Mix it with molten agar at about 45°C.
-
Pour into plates and allow it to solidify.
What you get:
-
Colonies grow on the surface and inside the agar.
-
Internal colonies look like tiny dots inside the medium.
Where it is useful:
-
Counting bacteria in water, milk, or food samples.
4. Lawn Culture (Confluent Culture)
What it is:
A method to grow bacteria as a uniform layer covering the entire plate.
How it is done:
-
Dip a sterile swab into the bacterial sample.
-
Spread it in 3 directions on the plate.
What you get:
A thick, complete growth of bacteria.
Why it is used:
-
Most commonly for antibiotic sensitivity testing (Kirby-Bauer test)
-
For bacteriophage typing
-
For testing bacteriocins
5. Stroke Culture
What it is:
Growing bacteria on the surface of an agar slant inside a test tube.
How it is done:
-
Use a loop.
-
Make a single straight streak from bottom to top on the slanted surface.
Why it is important:
-
Good for maintaining stock cultures.
-
Useful for enzyme tests.
-
Convenient for storing bacteria for short durations.
6. Stab Culture (Deep Culture)
What it is:
A method to grow bacteria inside the depth of semi-solid agar.
How it is done:
-
Use a straight wire (needle).
-
Push it straight into the agar tube, then pull it out.
What it helps to study:
-
Motility → motile bacteria spread away from the stab line.
-
Oxygen preference → some grow only at the top, some only in the middle.
-
H₂S production → blackening of the medium.
7. Anaerobic Culture Methods
What it is:
Techniques used to grow bacteria that cannot tolerate oxygen.
Common methods:
-
GasPak jar: creates oxygen-free environment.
-
Anaerobic chamber: special box with no oxygen.
-
Reducing media: like thioglycolate broth or Robertson’s cooked meat medium.
Why it is important:
To grow bacteria like:
-
Clostridium
-
Bacteroides
-
Other anaerobic pathogens
8. Liquid Culture (Broth Culture)
What it is:
Growing bacteria in nutrient broth, which is a liquid medium.
How it is done:
-
Add the inoculum into a tube containing broth.
-
Incubate at suitable temperature.
What you see:
-
Medium becomes cloudy (turbid) when bacteria grow.
-
Surface can show a pellicle or sediment at the bottom.
Why it is used:
-
To produce large numbers of bacteria.
-
For blood culture.
-
For enrichment before plating.
9. Enrichment Culture
What it is:
A method to increase the number of a particular type of bacteria when they are few in number.
How it is done:
-
Sample is added to an enrichment medium that helps the desired organism and inhibits others.
Examples:
-
Selenite F broth → helps Salmonella grow
-
Alkaline peptone water → helps Vibrio grow
Why it is important:
Useful when the pathogen is present in small numbers and can be easily missed.
10. Slide Culture (For Fungi)
What it is:
A special method to observe fungal structures (hyphae, spores) in their natural form.
How it is done:
-
A small cube of agar is placed on a slide.
-
Fungus is inoculated on the sides.
-
Covered and incubated.
What it shows:
Beautiful, clear arrangement of fungal structures.
Where it is used:
-
For molds like Aspergillus, Mucor, Penicillium.
11. Cell Culture (for Viruses)
What it is:
A method where living cells are used to grow viruses (because viruses cannot grow on agar or broth).
Types of cells used:
-
Primary cells – freshly isolated (short life)
-
Diploid cell lines – limited divisions
-
Continuous cell lines – immortal (e.g., Vero, HeLa)
Why it is needed:
-
Viral diagnosis
-
Vaccine production
-
Research work
12. Tissue or Organ Culture
What it is:
Growing small pieces of tissue or organs in a controlled environment.
Why used:
-
To study viruses that need organized tissue
-
To understand cell interactions
-
Research in virology and pathology
Summary Table
| Culture Method | Why It Is Used | Example Use |
|---|---|---|
| Streak plate | Isolation | Pure cultures |
| Spread plate | Uniform growth | CFU count |
| Pour plate | Enumeration | Food/water testing |
| Lawn culture | Confluent growth | Antibiotic sensitivity |
| Stroke culture | Stock growth | Biochemical tests |
| Stab culture | Motility & anaerobes | H₂S test |
| Anaerobic culture | Grow without oxygen | Clostridium |
| Broth culture | Large-scale growth | Blood culture |
| Enrichment culture | Boost target bacteria | Vibrio, Salmonella |
| Slide culture | Fungal ID | Molds |
| Cell/tissue culture | Virus growth | Virology |
General Steps for Preparation
- Weighing and Measuring:
- Use an analytical balance for accuracy. Commonly, the formulation for a litre of media is:
- For Nutrient Agar:
- Peptone: 5 g
- Beef extract: 3 g
- Agar: 15 g
- Distilled water: 1 L
- For Tryptic Soy Agar (TSA):
- Tryptone: 15 g
- Soy peptone: 5 g
- Agar: 15 g
- Distilled water: 1 L
- For Nutrient Agar:
- Use an analytical balance for accuracy. Commonly, the formulation for a litre of media is:
- Mixing:
- Dissolve the dry ingredients in the specified volume of distilled water in a flask or beaker. Stir gently to avoid foaming.
- Heating:
- Heat the mixture to boiling to dissolve the agar completely. Use a magnetic stirrer if available to ensure thorough mixing.
- pH Adjustment:
- After dissolving, allow the medium to cool slightly before measuring pH. Adjust using HCl or NaOH to reach the desired pH.
- Sterilization:
- Autoclave the media at 121°C for 15-20 minutes. Ensure that the autoclave is properly calibrated to avoid under- or over-sterilization.
- Cooling and Pouring:
- Allow the sterilized media to cool to 45-50°C before pouring to prevent condensation.
- Pour into sterile Petri dishes, filling to about 1/4 to 1/2 full for proper colony growth.
- Let the media solidify at room temperature.
Standardization of Culture Media
Standardization of culture media means ensuring that every batch of media prepared in the laboratory gives consistent, reliable, and reproducible results.
In simple words, the media should support the same level of microbial growth every time, without any variation.This is important because:
- Culture results must be accurate
- Diagnostic tests should be reproducible
- Bacteria should grow the same way each time
- Antibiotic sensitivity tests depend on media quality
- Poor-quality media can give false results
Standardization ensures that media is safe, effective, fresh, and uniform.
Why Standardization is Important
- To maintain uniform quality of media
- To avoid variations in test results
- To ensure correct pH, nutrient level, and sterility
- To ensure media supports expected bacterial growth
- To prevent contamination
- To follow international guidelines (CLSI, WHO)
Key Parameters in Standardization of Culture Media
Standardization involves checking the following:
1. Physical Properties
a) Appearance
- Media should have correct color and clarity
- No particles, no precipitation
b) Consistency
- Solid media should be firm
- Semi-solid media should have soft consistency
- Broth should be clear before inoculation
c) Gel Strength
- Agar must solidify properly
- Should not be too hard or too soft
2. pH Check
Correct pH ensures:
- Good microbial growth
- Proper biochemical reactions
Most media have pH between 7.0 and 7.4, but it varies depending on the medium.
3. Sterility Testing
Before using:
- Incubate a small amount of prepared media without inoculation
- Look for contamination for 24–48 hours
- If any growth appears → media is contaminated → discard
4. Growth Promotion Test (GPT)
This is the most important standardization step.
Purpose:
To check whether the medium can support the growth of microorganisms.
How it’s done:
- Inoculate the medium with standard strains
- Incubate for the required time
- Observe whether the bacteria grow as expected
Common control strains:
- Escherichia coli ATCC
- Staphylococcus aureus ATCC
- Pseudomonas aeruginosa ATCC
- Salmonella enterica ATCC
What is checked?
- Colony appearance
- Color change
- Hemolysis pattern
- Fermentation reaction
- Growth rate
If the results match the expected characteristics → media is good.
5. Selectivity Test (if selective media)
For selective media like MacConkey, MSA, TCBS:
- Check if target bacteria grow
- Check if non-target bacteria are inhibited
- Verify intensity of inhibition
Example:
MacConkey agar should inhibit Gram-positive bacteria.
6. Differential Property Test (if differential media)
For media with indicators:
- Check if color reactions are correct
- Check if lactose fermenters/non-fermenters behave normally
- Verify hemolysis on blood agar
This ensures that biochemical reactions are happening properly.
7. Storage and Shelf-Life Check
Proper labeling and storage conditions must be followed:
- Date of preparation
- Expiry date
- Storage temperature
- Batch number
Media should not:
- Dry out
- Melt
- Change color
- Lose moisture
Steps in Standardization (Simplified)
- Check physical quality (color, clarity, solidification)
- Measure pH
- Test sterility
- Perform growth promotion test
- Check selectivity (if selective medium)
- Check differential reactions (if applicable)
- Label, store, and maintain records
Common Problems Detected During Standardization
| Problem | Possible Cause |
|---|---|
| Poor growth | Wrong pH, expired ingredients, improper sterilization |
| Excessive moisture | Hot pouring, poor storage |
| Soft agar | Low agar content |
| Hard agar | Too much agar |
| Color change | pH change or contamination |
| Unexpected reactions | Indicator not working or wrong preparation |
MCQs
-
The main purpose of culture media is to:
A) Kill microbes B) Grow microbes C) Dry microbes D) Stain microbes
Ans: B -
The primary solidifying agent used in culture media is:
A) Gelatin B) Agar C) Starch D) Pectin
Ans: B -
Agar is obtained from:
A) Bacteria B) Seaweed C) Mushrooms D) Fungi
Ans: B -
The melting point of agar is around:
A) 40°C B) 65°C C) 90–100°C D) 25°C
Ans: C -
The solidification temperature of agar is around:
A) 0°C B) 45°C C) 70°C D) 100°C
Ans: B -
Liquid media are also known as:
A) Slants B) Broths C) Plates D) Tubes
Ans: B -
Semi-solid media contain approximately:
A) 1% agar B) 0.5% agar C) 5% agar D) No agar
Ans: B -
Nutrient agar is an example of:
A) Synthetic medium B) Enriched medium C) Simple medium D) Selective medium
Ans: C -
Beef extract in media provides:
A) Water B) Carbs C) Vitamins and minerals D) Lipids
Ans: C -
Peptones provide:
A) Fats B) Protein fragments C) Carbohydrates D) Metals
Ans: B -
A medium with known chemical composition is called:
A) Simple B) Defined C) Complex D) Enriched
Ans: B -
Complex media contain:
A) Exact known chemicals B) Unknown nutrient mixtures C) Only pure chemicals D) No proteins
Ans: B -
The most commonly used pH for bacterial culture media is:
A) 2.0 B) 5.5 C) 7.0–7.4 D) 9.0
Ans: C -
Agar is preferred because it is:
A) Digestible B) Cheap and edible C) Not digested by most bacteria D) Solid at 100°C
Ans: C -
Media used to maintain viability during transport is:
A) Enriched B) Transport C) Selective D) Differential
Ans: B -
Stuart’s medium is a:
A) Enrichment medium B) Transport medium C) Differential medium D) Complex medium
Ans: B -
MacConkey agar inhibits:
A) Gram-negative bacteria B) Gram-positive bacteria C) Mycobacteria D) Anaerobes
Ans: B -
Blood agar shows:
A) Motility B) Hemolysis C) Pigment formation D) Sporulation
Ans: B -
Thiosulfate citrate bile salt sucrose (TCBS) agar is used for:
A) E. coli B) Vibrio C) Streptococci D) Mycobacteria
Ans: B -
Lowenstein–Jensen medium is used for:
A) Fungi B) Mycobacteria C) Streptococci D) Viruses
Ans: B -
A medium that supports growth of fastidious organisms is:
A) Transport B) Enriched C) Selective D) Synthetic
Ans: B -
Chocolate agar is prepared by heating:
A) Peptone broth B) Nutrient agar C) Blood agar D) Gelatin
Ans: C -
Differential media help to:
A) Kill bacteria B) Highlight biochemical differences C) Store cultures D) Fix organisms
Ans: B -
Indicator used in MacConkey agar:
A) Phenol red B) Neutral red C) Crystal violet D) Methylene blue
Ans: B -
Lactose fermenters appear on MacConkey agar as:
A) Colorless colonies B) Black colonies C) Pink colonies D) Blue colonies
Ans: C -
Mannitol salt agar selects:
A) Streptococci B) Neisseria C) Staphylococci D) E. coli
Ans: C -
High salt concentration in MSA is:
A) 1% B) 2% C) 7.5% D) 0.5%
Ans: C -
CLED agar is commonly used in:
A) Stool culture B) Urine culture C) Blood culture D) Mycology
Ans: B -
Sabouraud’s dextrose agar is used for culturing:
A) Viruses B) Fungi C) Parasites D) Anaerobes
Ans: B -
Robertson’s cooked meat (RCM) medium is used for:
A) Aerobes B) Fungi C) Anaerobes D) Viruses
Ans: C -
Selenite F broth is a selective enrichment medium for:
A) E. coli B) Salmonella C) Vibrio D) Staphylococcus
Ans: B -
Alkaline peptone water enriches:
A) Shigella B) Staphylococcus C) Vibrio D) Streptococcus
Ans: C -
EMB agar differentiates:
A) Gram-positive cocci B) Gram-negative bacilli C) Fungi D) Mycobacteria
Ans: B -
E. coli shows a characteristic metallic sheen on ______ agar.
A) Blood B) MacConkey C) EMB D) CLED
Ans: C -
Media for anaerobes must contain:
A) Oxygen B) Reducing agents C) High glucose D) Alcohol
Ans: B -
Which medium uses bile salts as inhibitors?
A) Blood agar B) MacConkey agar C) SDA D) LJ medium
Ans: B -
Minimal media are examples of:
A) Defined media B) Complex media C) Enriched media D) Transport media
Ans: A -
XLD agar is used for isolating:
A) Vibrio B) Pseudomonas C) Salmonella & Shigella D) Streptococcus
Ans: C -
TSI medium tests:
A) Motility B) Carbohydrate fermentation & H₂S C) Pigmentation D) Spore formation
Ans: B -
Simmons citrate medium tests for:
A) Urease B) Citrate utilization C) Lactose fermentation D) Indole
Ans: B -
Most media are sterilized by:
A) Dry heat B) Autoclaving C) Filtration D) UV light
Ans: B -
Autoclave temperature for sterilizing media:
A) 100°C B) 121°C C) 150°C D) 80°C
Ans: B -
Autoclave pressure used:
A) 5 psi B) 10 psi C) 15 psi D) 25 psi
Ans: C -
Agar plates should be stored:
A) Upright B) Inverted C) Open D) At −20°C
Ans: B -
Heat-sensitive media are sterilized by:
A) Boiling B) UV light C) Filtration D) Autoclave
Ans: C -
The pH of media is measured using:
A) Balance B) pH meter C) Microscope D) Oven
Ans: B -
Media with blood are sterilized by:
A) Autoclaving B) Filtration C) Inspissation D) UV
Ans: C -
The process of pouring plates is done at:
A) 90°C B) 45–50°C C) 20°C D) 0°C
Ans: B -
Agar that is too soft results from:
A) Excess agar B) Low agar concentration C) Old media D) High pH
Ans: B -
Media must be stored typically at:
A) 0°C B) 2–8°C C) 40°C D) 100°C
Ans: B -
Sterility testing involves:
A) Adding bacteria B) Incubating uninoculated media C) Using dyes D) Measuring moisture
Ans: B -
Unwanted precipitation in media indicates:
A) Good quality B) pH problems C) Correct sterilization D) Ideal incubation
Ans: B -
Gel strength depends on:
A) Agar content B) pH C) Temperature D) Water hardness
Ans: A -
Growth promotion test checks:
A) Moisture B) Growth of known strains C) Chemical purity D) Sterility only
Ans: B -
Sterile media without growth is:
A) Positive control B) Negative control C) Growth control D) Indicator
Ans: B -
Slants are made by cooling tubes at:
A) Horizontal angle B) Vertical angle C) No angle D) In fridge
Ans: A -
Dehydrated media powder must be stored:
A) In sunlight B) In humid area C) In dry, cool place D) In freezer
Ans: C -
Colony morphology cannot be studied on:
A) Plates B) Slants C) Broth D) SDA plates
Ans: C -
Stock cultures are usually kept on:
A) Broths B) Slants C) Plates D) Tissue culture
Ans: B -
A medium turning yellow after autoclaving may indicate:
A) Ideal pH B) pH drop C) Extra agar D) Sterility loss
Ans: B -
Media used for urine culture:
A) XLD B) CLED C) TCBS D) LJ
Ans: B -
MSA contains indicator:
A) Phenol red B) Neutral red C) Methyl red D) Crystal violet
Ans: A -
Chocolate agar is brown due to:
A) Caramel B) Lysis of RBCs C) Fungal pigment D) Oxidation
Ans: B -
Blood agar detects:
A) Spore formation B) Hemolysis C) Capsule D) Motility
Ans: B -
α-hemolysis shows:
A) Clear zone B) Greenish zone C) No zone D) Black zone
Ans: B -
β-hemolysis shows:
A) Greenish zone B) Black zone C) Clear zone D) No change
Ans: C -
γ-hemolysis means:
A) Complete lysis B) Partial lysis C) No lysis D) Blackening
Ans: C -
Media that differentiate lactose fermenters:
A) SDA B) MacConkey C) LJ D) RCM
Ans: B -
Hektoen enteric agar is used for:
A) Gonococci B) Mycobacteria C) Enteric pathogens D) Fungi
Ans: C -
Cary-Blair medium is:
A) Transport medium B) Differential medium C) Selective medium D) Anaerobic medium
Ans: A -
Selenite F broth suppresses:
A) Salmonella B) E. coli C) Vibrio D) Pseudomonas
Ans: B -
XLD agar contains indicators for:
A) Citrate B) Hemolysis C) Sugar fermentation & H₂S D) Motility
Ans: C -
Brilliant green agar grows mainly:
A) Salmonella B) Streptococci C) Vibrio D) Fungi
Ans: A -
Urea agar tests for:
A) Lactose fermentation B) Urease enzyme C) Motility D) Oxidase
Ans: B -
Simmons citrate medium turns blue when:
A) Citrate used B) Glucose used C) Lactose used D) H₂S formed
Ans: A -
A medium that contains blood is:
A) Synthetic B) Enriched C) Selective D) Minimal
Ans: B -
Mycology media like SDA are acidic at pH:
A) 5.6 B) 7.0 C) 8.2 D) 9.0
Ans: A -
Media for virus isolation:
A) Blood agar B) Cell culture medium C) MacConkey D) EMB
Ans: B -
Anaerobes are cultured in:
A) GasPak jar B) Candle jar C) UV cabinet D) Refrigerator
Ans: A -
Thioglycolate broth produces:
A) Oxygen B) Reduction of oxygen C) High salt D) Acid only
Ans: B -
Most common method for isolating pure colonies:
A) Spread plate B) Streak plate C) Pour plate D) Slant
Ans: B -
Spread plate is mainly used for:
A) Hemolysis B) CFU counting C) Motility D) Acid production
Ans: B -
Pour plate allows bacteria to grow:
A) Only on surface B) Only inside agar C) Both inside and surface D) Only at edges
Ans: C -
Lawn culture produces:
A) Individual colonies B) Confluent growth C) Dry colonies D) Liquid growth
Ans: B -
Stab culture is ideal for:
A) Hemolysis B) Motility C) Capsule D) Pigment
Ans: B -
Stroke culture is mostly used for:
A) Stock maintenance B) Antibiotic testing C) Motility D) Urine culture
Ans: A -
Bordet-Gengou medium is used for:
A) TB B) Whooping cough organism C) Fungi D) Enterococci
Ans: B -
Chocolate agar supports growth of:
A) Haemophilus B) Vibrio C) Fungi D) Salmonella
Ans: A -
Media for Neisseria:
A) TCBS B) Thayer-Martin agar C) CLED D) Blood agar only
Ans: B -
Triple sugar iron (TSI) detects:
A) H₂S production B) Capsule C) Spores D) Viral inclusions
Ans: A -
Motility is best seen in:
A) Solid agar B) Semi-solid agar C) Liquid broth only D) Chocolate agar
Ans: B -
Media with oxygen indicator:
A) MacConkey B) Thioglycolate C) LJ medium D) EMB
Ans: B -
A medium that both selects and differentiates is:
A) Blood agar B) MacConkey agar C) SDA D) Broth
Ans: B -
Selective media inhibit:
A) All bacteria B) Only target bacteria C) Unwanted bacteria D) Fungi
Ans: C -
Fastidious organisms require:
A) Extra nutrients B) Less nutrients C) No nutrients D) Sunlight
Ans: A -
A medium containing 2 or more indicators is:
A) Selective B) Differential C) Enriched D) Transport
Ans: B -
Capnophilic organisms grow well in:
A) Nitrogen B) High CO₂ C) High O₂ D) Low temp
Ans: B -
The primary medium for routine bacterial culture is:
A) Blood agar B) CLED C) LJ medium D) TCBS
Ans: A -
Shelf life of culture plates decreases when:
A) Stored inverted B) Excess moisture present C) Kept at 4°C D) Labelled correctly
Ans: B -
Quality control of culture media includes:
A) Only autoclaving
B) Only pH checking
C) Growth promotion test, sterility test, pH, physical check
D) No testing required
Ans: C