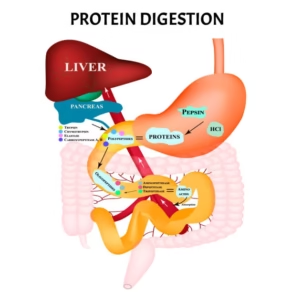
Introduction
- Proteins are fundamental macronutrients that form the structural and functional framework of life.
- Every living cell depends on proteins for enzymatic reactions, signaling, contractility, transport, and immune protection.
- Dietary proteins are polymers of amino acids linked by peptide bonds and must be hydrolyzed into smaller absorbable units before utilization.
- Unlike carbohydrates and lipids, which can be partially absorbed in simpler forms, proteins cannot cross the intestinal mucosa intact (except in newborns).
- Therefore, digestion and absorption of proteins are complex processes involving multiple organs, specialized enzymes, and regulated transport systems that ensure complete hydrolysis and efficient assimilation of amino acids.
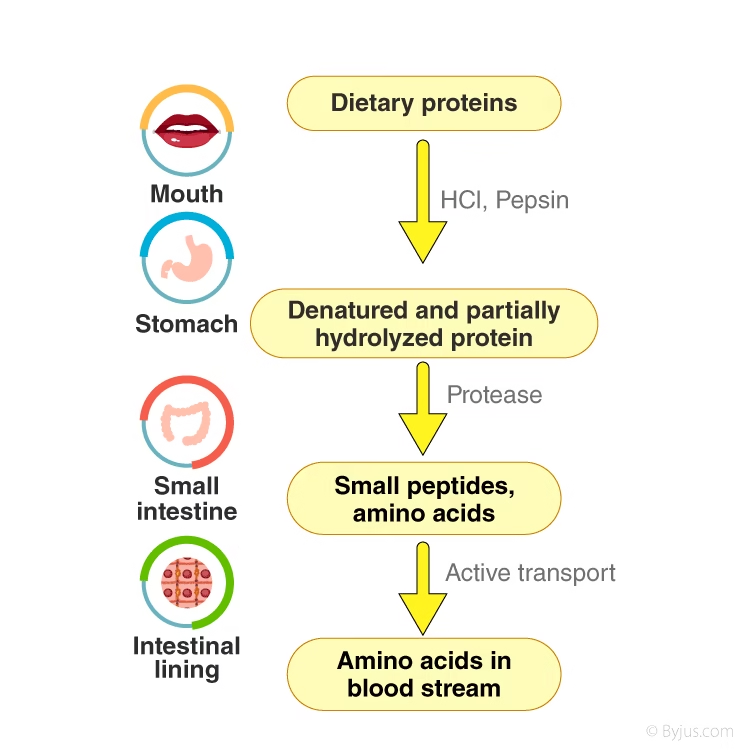
The process can be divided into the following sequential stages:
-
Ingestion and denaturation (in the stomach)
-
Enzymatic hydrolysis (pancreatic and intestinal phases)
-
Absorption of amino acids and peptides (intestinal mucosa)
-
Transport to the liver and systemic circulation
Protein Digestion
Protein digestion is a multistep enzymatic process involving cooperation among the stomach, pancreas, and small intestine.
| Phase | Site of Action | Major Enzymes | Optimum pH | End Products |
|---|---|---|---|---|
| Gastric Phase | Stomach | Pepsin | 1.5–2.5 | Polypeptides, Peptones |
| Pancreatic Phase | Duodenum | Trypsin, Chymotrypsin, Elastase, Carboxypeptidase | 7.5–8.5 | Oligopeptides |
| Intestinal Phase | Jejunum, Ileum | Aminopeptidase, Dipeptidase, Tripeptidase | 7.5–8.0 | Free amino acids, small peptides |
Gastric Digestion
Gastric Juice Composition
-
Hydrochloric acid (HCl) from parietal cells – provides low pH for enzyme activation.
-
Pepsinogen from chief cells – converted to active pepsin.
-
Mucus – protects the gastric mucosa from autodigestion.
-
Intrinsic factor – essential for vitamin B₁₂ absorption (not directly protein digestion but part of gastric function).
Mechanism of Action
-
Hydrochloric acid (HCl) in the stomach breaks the complex shape of proteins by unfolding their tertiary and quaternary structure.
-
This process denatures hydrogen and ionic bonds, exposing the peptide bonds inside the protein chain.
-
Pepsinogen, an inactive enzyme released by chief cells, is activated by HCl to form pepsin.
-
Pepsin is an endopeptidase that cuts large protein molecules into smaller pieces called polypeptides and peptones.
-
Pepsin mainly cuts peptide bonds near aromatic amino acids (phenylalanine, tyrosine, tryptophan) and acidic residues (aspartate, glutamate).
-
The result of this stage is a partially digested mixture (chyme) that moves to the small intestine for further digestion.
Regulation of the Gastric Phase
-
Cephalic Phase:
-
Triggered by the sight, smell, or taste of food.
-
The vagus nerve releases acetylcholine (ACh), stimulating gastric glands to secrete acid and enzymes before food enters the stomach.
-
-
Gastric Phase:
-
Starts when food enters the stomach and causes stretching (distension).
-
Peptides from food stimulate G cells in the stomach lining to release gastrin.
-
Gastrin increases secretion of HCl (from parietal cells) and pepsinogen (from chief cells).
-
-
Inhibitory Control:
-
When the pH becomes too low (<2), somatostatin is released.
-
Somatostatin and the low acidity suppress gastrin release, preventing excess acid formation.
-
Clinical Note
-
Deficiency of gastric acid (achlorhydria) leads to hypoproteinemia due to impaired digestion.
-
Pepsin is inactive above pH 5.0, so protein digestion ceases in the duodenum until pancreatic enzymes act.
Pancreatic Digestion
After the gastric phase, the acidic chyme enters the duodenum, where bicarbonate (HCO₃⁻) from the pancreas neutralizes the acid. This alkaline environment (pH 7.5–8.5) is optimal for pancreatic proteases.
Pancreatic Enzyme Secretion
Pancreatic acinar cells secrete a mixture of zymogens (inactive precursors) to prevent self-digestion. These zymogens are activated only in the intestinal lumen.
| Zymogen (Inactive form) | Active Enzyme | Activator | Major Substrate Bonds Cleaved |
|---|---|---|---|
| Trypsinogen | Trypsin | Enteropeptidase | Lysine, Arginine |
| Chymotrypsinogen | Chymotrypsin | Trypsin | Aromatic AAs (Phe, Tyr, Trp) |
| Proelastase | Elastase | Trypsin | Small neutral AAs (Ala, Gly, Val) |
| Procarboxypeptidase A/B | Carboxypeptidase A/B | Trypsin | C-terminal residues (A or basic AAs) |
Activation Cascade
-
Enteropeptidase (Enterokinase), a brush border enzyme, converts trypsinogen → trypsin.
-
Trypsin then autocatalytically activates remaining trypsinogen and other zymogens (chymotrypsinogen, proelastase, procarboxypeptidase).
-
This cascade ensures amplification and tight regulation.
Role of Pancreatic Enzymes
-
Endopeptidases (trypsin, chymotrypsin, elastase) act within the polypeptide chain.
-
Exopeptidases (carboxypeptidases A and B) remove amino acids sequentially from the carboxyl terminal end.
Importance of pH Regulation
- Bicarbonate from the pancreas maintains an alkaline pH, which is necessary for enzyme stability.
- If bicarbonate secretion fails (e.g., in cystic fibrosis), the intestinal lumen becomes acidic, inactivating enzymes and leading to malabsorption.
Intestinal Digestion
The final digestion occurs at the brush border membrane of enterocytes (intestinal epithelial cells).
Major Brush Border Peptidases
| Enzyme | Action | Products Formed |
|---|---|---|
| Aminopeptidase | Removes amino acids from N-terminus | Shorter peptides, amino acids |
| Dipeptidase / Tripeptidase | Splits di- and tripeptides | Free amino acids |
| Enteropeptidase | Converts trypsinogen → trypsin | Initiates activation cascade |
These enzymes complete the hydrolysis, producing free amino acids, dipeptides, and tripeptides suitable for absorption.
Cytoplasmic Peptidases
Once di- and tripeptides enter enterocytes, cytosolic peptidases hydrolyze them further, ensuring that almost all absorbed nitrogen is in the form of free amino acids before entering the portal circulation.
Absorption of Amino Acids and Peptides
- Protein absorption occurs primarily in the jejunum and ileum via specific membrane transporters.
- It involves both active transport (energy-requiring) and facilitated diffusion mechanisms.
Amino Acid Transport Systems
| System Name | Main Amino Acids Transported | Mechanism | Location | Clinical Significance / Example Disorder |
|---|---|---|---|---|
| System A | Small neutral amino acids – Alanine, Serine, Glycine | Na⁺-dependent active transport | Intestinal mucosa and renal tubules | Major route for uptake of neutral amino acids; a defect can cause Hartnup disease (neutral aminoaciduria). |
| System X⁻AG | Acidic amino acids – Aspartate, Glutamate | Na⁺-dependent cotransport | Brush the border of the intestine, the kidney | Responsible for acidic amino acid reabsorption; defect → dicarboxylic aminoaciduria. |
| System L | Large neutral amino acids – Leucine, Isoleucine, Phenylalanine, Tyrosine | Facilitated diffusion (Na⁺-independent) | The basolateral membrane of enterocytes and most tissues | Important for amino acid exchange between plasma and cells; linked with muscle uptake and amino acid transport into the brain. |
| System y⁺ | Basic amino acids – Lysine, Arginine, Ornithine | Na⁺-independent carrier | Intestine, kidney, liver | Defective in Cystinuria, leading to cystine stones and dibasic aminoaciduria. |
| System IMINO | Proline and Hydroxyproline (imino acids) | Na⁺-linked transport | Small intestine and renal tubules | Specialized system for cyclic imino acids; defect → Iminoglycinuria (loss of proline, hydroxyproline, glycine). |
Each transporter is stereospecific (L-amino acids are preferentially absorbed).
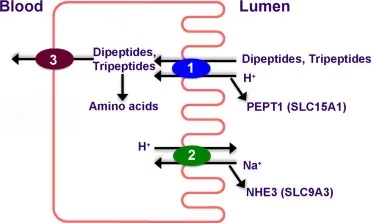
Peptide Transport System (PEPT1)
- The PEPT1 transporter is a H⁺-coupled symporter that transports dipeptides and tripeptides into enterocytes.
- Inside the cell, they are hydrolyzed to free amino acids by intracellular peptidases.
This mechanism enables the rapid and efficient absorption of dietary nitrogen, as dipeptides are absorbed more readily than free amino acids.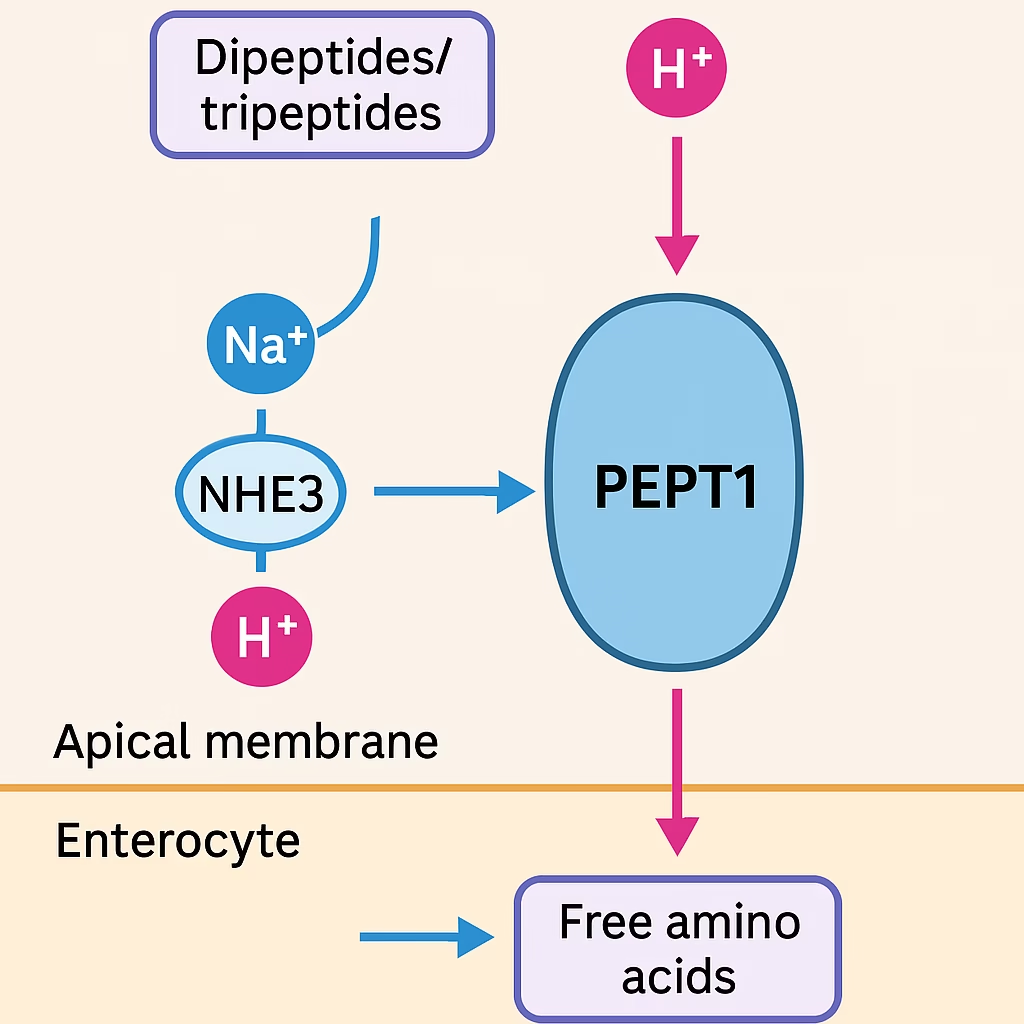
Basolateral Transport to Blood
- After absorption, amino acids exit the enterocyte through the basolateral membrane via Na⁺-independent facilitated diffusion into the portal vein, which carries them to the liver.
- The liver regulates plasma amino acid concentration through deamination, transamination, and urea cycle pathways.
Regulation of Protein Digestion
Protein digestion and absorption are tightly coordinated by neural and hormonal factors.
| Regulator | Source | Primary Function |
|---|---|---|
| Gastrin | G cells (stomach) | Stimulates HCl and pepsinogen release |
| Secretin | Duodenal mucosa | Triggers pancreatic bicarbonate secretion |
| Cholecystokinin (CCK) | Intestinal mucosa | Stimulates pancreatic enzyme and bile release |
| Somatostatin | D cells | Inhibits gastrin and acid secretion |
| Vagus nerve (ACh) | Parasympathetic fibers | Enhances gastric and intestinal motility |
This integration ensures efficient enzyme secretion only when dietary proteins are present.
Clinical Aspects and Disorders
| Condition | Defect / Mechanism | Clinical Features |
|---|---|---|
| Celiac Disease | Gluten-induced villous atrophy | Steatorrhea, malabsorption, anemia |
| Cystinuria | Defective basic AA transporter (y⁺ system) | Cystine stones, aminoaciduria |
| Hartnup Disease | Neutral AA transport defect (system A) | Pellagra-like rash, ataxia, aminoaciduria |
| Pancreatic Insufficiency | Decreased enzyme secretion (CF, pancreatitis) | Protein maldigestion, steatorrhea |
| Tropical Sprue | Mucosal damage due to infection | Malabsorption, diarrhea, weight loss |
| Kwashiorkor / Marasmus | Protein-energy malnutrition | Growth failure, edema, muscle wasting |
Diagnostic Evaluation
-
Stool nitrogen test – indicates undigested protein loss.
-
Fecal elastase assay – marker for pancreatic function.
-
Serum trypsinogen – low in pancreatic insufficiency.
-
D-xylose absorption test – differentiates mucosal vs. pancreatic defect.
Hepatic Utilization of Absorbed Amino Acids
Once amino acids reach the liver, they enter several metabolic fates:
-
Protein synthesis (plasma proteins, enzymes, hormones).
-
Transamination to form non-essential amino acids.
-
Deamination and oxidative breakdown for energy.
-
Ammonia detoxification through the urea cycle.
-
Conversion to glucose or ketone bodies in starvation.
Thus, the liver acts as the metabolic hub of nitrogen homeostasis.
MCQs
-
The digestion of proteins begins in the:
A) Mouth
B) Stomach
C) Duodenum
D) Jejunum -
The enzyme pepsin is secreted in which form?
A) Pepsin
B) Pepsinogen
C) Peptidase
D) Peptide -
The pH optimum for pepsin activity is:
A) 1.5–2.5
B) 4–5
C) 6–7
D) 8–9 -
Pepsin is classified as a/an:
A) Exopeptidase
B) Endopeptidase
C) Dipeptidase
D) Carboxypeptidase -
Hydrochloric acid in the stomach mainly:
A) Activates trypsin
B) Denatures proteins
C) Converts amino acids into glucose
D) Hydrolyzes fats -
Which hormone stimulates gastric acid secretion?
A) Secretin
B) Cholecystokinin
C) Gastrin
D) Somatostatin -
Pepsin primarily cleaves peptide bonds next to:
A) Sulfur amino acids
B) Aromatic amino acids
C) Basic amino acids
D) Branched-chain amino acids -
Chief cells of the stomach secrete:
A) HCl
B) Pepsinogen
C) Mucus
D) Gastrin -
Which nerve mediates the cephalic phase of gastric secretion?
A) Vagus nerve
B) Phrenic nerve
C) Glossopharyngeal nerve
D) Hypoglossal nerve -
Gastric proteolysis stops when chyme enters the:
A) Colon
B) Duodenum
C) Ileum
D) Rectum
-
Trypsinogen is activated by:
A) Pepsin
B) Enteropeptidase
C) Elastase
D) Amylase -
Trypsin activates all except:
A) Chymotrypsinogen
B) Proelastase
C) Procarboxypeptidase
D) Pepsinogen -
Pancreatic enzymes act best at pH:
A) 2–3
B) 5–6
C) 7.5–8.5
D) 9–10 -
The enzyme that acts on aromatic amino acids is:
A) Trypsin
B) Chymotrypsin
C) Elastase
D) Carboxypeptidase B -
Carboxypeptidase A removes:
A) N-terminal amino acids
B) C-terminal aromatic amino acids
C) C-terminal basic amino acids
D) Internal peptide bonds -
Enteropeptidase is secreted by:
A) Pancreas
B) Liver
C) Intestinal mucosa
D) Stomach -
Which enzyme hydrolyzes bonds next to small neutral amino acids?
A) Trypsin
B) Chymotrypsin
C) Elastase
D) Pepsin -
Failure of bicarbonate secretion in cystic fibrosis leads to:
A) Increased enzyme activity
B) Inactivation of pancreatic enzymes
C) Decreased pepsin secretion
D) Enhanced absorption -
The inactive precursor of trypsin is called:
A) Pepsinogen
B) Trypsinogen
C) Chymotrypsinogen
D) Proelastase -
Which pancreatic enzyme is an exopeptidase?
A) Trypsin
B) Chymotrypsin
C) Carboxypeptidase
D) Elastase
-
Brush-border enzymes include all except:
A) Aminopeptidase
B) Dipeptidase
C) Pepsin
D) Enteropeptidase -
Final digestion of proteins occurs in the:
A) Duodenum
B) Jejunum
C) Colon
D) Mouth -
Aminopeptidase removes amino acids from:
A) C-terminal
B) N-terminal
C) Middle of chain
D) Aromatic side chain -
PEPT1 transports:
A) Free amino acids
B) Dipeptides and tripeptides
C) Monosaccharides
D) Fatty acids -
PEPT1 transport is driven by:
A) Na⁺ gradient
B) H⁺ gradient
C) ATP directly
D) Cl⁻ transport -
The Na⁺/H⁺ exchanger maintains:
A) Sodium balance only
B) Hydrogen ion gradient for PEPT1
C) Chloride secretion
D) Calcium absorption -
After entering enterocytes, dipeptides are:
A) Stored
B) Excreted
C) Hydrolyzed to free amino acids
D) Transported intact to liver -
Amino acid absorption occurs mainly in:
A) Stomach
B) Duodenum
C) Jejunum and ileum
D) Colon -
Transport of basic amino acids occurs via:
A) System L
B) System A
C) System y⁺
D) System IMINO -
Defect in neutral amino acid transport causes:
A) Cystinuria
B) Hartnup disease
C) Celiac disease
D) Marasmus
-
Amino acids exit enterocytes through which membrane?
A) Apical
B) Basolateral
C) Luminal
D) Nuclear -
Basolateral amino acid transport is mostly:
A) Na⁺-dependent
B) Facilitated diffusion (Na⁺-independent)
C) Active transport
D) Pinocytosis -
Portal blood carries amino acids directly to:
A) Heart
B) Kidney
C) Liver
D) Spleen -
The hormone stimulating pancreatic enzyme secretion is:
A) Gastrin
B) Secretin
C) CCK
D) Somatostatin -
Secretin primarily causes:
A) Pepsin release
B) Bicarbonate secretion
C) Lipase activation
D) HCl formation -
CCK stimulates:
A) Pancreatic enzyme and bile release
B) Acid formation in stomach
C) Urea cycle in liver
D) Glucose reabsorption -
Somatostatin acts as:
A) Stimulator
B) Inhibitor of gastric and pancreatic secretion
C) Activator of gastrin
D) Hormone of acid release -
In newborns, intact proteins are absorbed by:
A) Diffusion
B) Pinocytosis
C) Osmosis
D) PEPT1 system -
Failure of pancreatic enzymes causes:
A) Hypoglycemia
B) Steatorrhea and protein malabsorption
C) Hyperlipidemia
D) Diuresis -
Defect in basic amino acid transporter causes:
A) Cystinuria
B) Hartnup disease
C) Celiac disease
D) Scurvy
✅ Answer Key
| Q.No | Answer | Q.No | Answer | Q.No | Answer | Q.No | Answer |
|---|---|---|---|---|---|---|---|
| 1 | B | 11 | B | 21 | C | 31 | B |
| 2 | B | 12 | D | 22 | B | 32 | B |
| 3 | A | 13 | C | 23 | B | 33 | C |
| 4 | B | 14 | B | 24 | B | 34 | C |
| 5 | B | 15 | B | 25 | B | 35 | B |
| 6 | C | 16 | C | 26 | B | 36 | A |
| 7 | B | 17 | C | 27 | C | 37 | B |
| 8 | B | 18 | B | 28 | C | 38 | B |
| 9 | A | 19 | B | 29 | C | 39 | B |
| 10 | B | 20 | C | 30 | B | 40 | A |