Antigens
- Antigens are molecules that the immune system identifies as foreign, prompting it to initiate a response to neutralize or eliminate the perceived threat.
- Antigens play a crucial role in the body’s defense mechanisms by alerting immune cells to the presence of pathogens, such as bacteria, viruses, fungi, and other foreign substances.

- They can also include substances like toxins, transplanted organs, and pollen.
General Characteristics and Nature of Antigens
- Chemical Composition: Most antigens are proteins or polysaccharides, but they can also be lipids or nucleic acids in some cases. Due to their structural complexity, proteins are the most immunogenic (likely to provoke an immune response).
- Molecular Size: Antigens are typically large molecules with a molecular weight above 10,000 Daltons. Larger, more complex molecules tend to be more immunogenic.
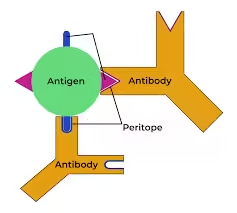
- Epitopes: Antigens contain specific regions called epitopes, or antigenic determinants, which are the exact parts of the antigen recognized by immune cells and antibodies. A single antigen can have multiple epitopes, each capable of binding to different antibodies.
- Foreignness: The immune system typically recognizes molecules as antigens only if they are “non-self,” meaning foreign to the body. However, in autoimmune conditions, self-antigens can also trigger immune responses.
Types of Antigens
Antigens can be classified based on their origin, function, and the type of immune response they evoke:
- Based on Origin:
- Exogenous Antigens: These come from outside the body, entering through inhalation, ingestion, or contact. Examples include microbes (bacteria, viruses, fungi), toxins, and allergens like pollen.
- Endogenous Antigens: These are produced within cells, typically due to viral or bacterial infections. Infected cells present fragments of these antigens on their surfaces, signaling for immune destruction.
- Autoantigens: Self-antigens are normally tolerated by the immune system but may become targets in autoimmune diseases, leading to an immune response against the body’s cells.
- Tumor Antigens: Abnormal or altered versions of normal proteins specific to cancerous cells. These antigens help the immune system target and destroy tumor cells.
- Based on Function:
- Complete Antigens: Molecules that can provoke an immune response and bind directly with immune receptors (e.g., B cells and T cells).
- Haptens: Small molecules that cannot trigger an immune response but can become immunogenic when attached to a larger carrier molecule, such as a protein.
- Based on Immune Response:
- T-Dependent Antigens: Require assistance from T-helper cells to activate B cells and induce antibody production. These include most protein antigens.
- T-Independent Antigens: Can directly stimulate B cells without T-helper cells’ involvement, often polysaccharides and other large molecules.
Role of Antigens in the Immune System
Antigens play a fundamental role in immune surveillance and defense by:
- Triggering Immune Responses: Upon recognizing an antigen, the immune system activates lymphocytes, including B cells (for antibody production) and T cells (for direct attack or support of other immune cells).
- Activating Antigen-Presenting Cells (APCs): Cells like dendritic cells and macrophages capture and process antigens, presenting them on their surfaces in conjunction with MHC molecules. This presentation is key to activating T cells, especially in adaptive immunity.
- Stimulating Antibody Production: When B cells recognize an antigen, they proliferate and differentiate into plasma cells that produce antibodies specific to that antigen, marking it for destruction or neutralizing its effects.
- Building Immunological Memory: After initial exposure to an antigen, the immune system retains a memory of it through memory B and T cells, enabling a faster and stronger response upon re-exposure to the same antigen.
Antibodies
- Antibodies are Y-shaped proteins that circulate through the bloodstream and lymphatic system, ready to identify and bind to specific antigens foreign to the body.
- Each antibody is highly specific to a particular antigen due to the unique structure of its antigen-binding site.
- This specificity enables antibodies to recognize and neutralize a wide range of pathogens.
- Upon antigen binding, antibodies can neutralize pathogens directly or flag them for destruction by other immune cells.
General Characteristics and Structure of Antibodies
Antibodies share a common structural framework but vary significantly in their antigen-binding sites, allowing for specificity to diverse antigens.
- Y-Shaped Structure: Each antibody molecule has a distinctive Y-shaped structure composed of four polypeptide chains—identical heavy chains and two identical light chains—held together by disulfide bonds.
- Variable and Constant Regions:
- Variable (V) Regions: Located at the tips of the Y-shaped structure, these regions are unique to each antibody and contain antigen-binding sites. The amino acid sequences in the variable regions vary greatly between antibodies, enabling specificity for various antigens.
- Constant (C) Regions: Found in the stem (Fc region) and lower parts of the arms of the Y, these regions are less variable and are associated with the antibody class. They determine how the antibody interacts with immune cells and molecules in the immune system, such as complement proteins.
- Antigen-Binding Sites: Each antibody has two antigen-binding sites that are highly specific for a particular epitope on an antigen. The binding of an antigen to an antibody is similar to a lock-and-key fit, providing specificity to the immune response.
- Flexibility and Functional Adaptability: The hinge region of the antibody allows the Y-shaped arms to move, increasing the flexibility of the antibody and its ability to interact with multiple epitopes on the same or different antigens.
Types of Antibodies (Immunoglobulins)
Antibodies are divided into five main classes—IgG, IgM, IgA, IgE, and IgD—based on their structure, location, and role in the immune response. Each class has a unique function and distribution in the body.
- IgG (Immunoglobulin G):
- The most abundant antibody in the bloodstream and extracellular fluid, IgG, accounts for approximately 70-75% of serum antibodies.
- IgG can cross the placenta, providing passive immunity to the fetus.
- Functions include neutralizing pathogens, opsonizing (coating) microbes for easier phagocytosis, and activating the complement system.
- IgG is critical in long-term immunity and is the primary antibody produced during a secondary immune response.
- IgM (Immunoglobulin M):
- The largest antibody, IgM, is the first antibody produced in response to an infection and is highly effective in early immune responses.
- Found mainly in the blood and lymph fluid, it forms pentamers (five Y-shaped units), making it very effective at agglutinating (clumping) pathogens and activating the complement system.
- IgM provides initial immune protection before IgG antibodies are produced.
- IgA (Immunoglobulin A):
- Found primarily in mucous membranes and secretions, such as saliva, tears, and breast milk, IgA protects mucosal surfaces from infections.
- IgA exists primarily as a dimer (two Y-shaped units linked together) in secretions, which helps it remain stable and effective in hostile environments like the gastrointestinal and respiratory tracts.
- IgA is particularly important in defending against pathogens entering through mucosal surfaces.
- IgE (Immunoglobulin E):
- IgE is involved in allergic reactions and defense against parasitic infections.
- It binds to basophils and mast cells, which release histamine and other chemicals upon antigen binding, leading to inflammation and allergic symptoms.
- Though present in low concentrations, IgE plays a significant role in immune responses to allergens and parasites.
- IgD (Immunoglobulin D):
- Found mainly on the surface of immature B cells, IgD acts as a receptor for antigens and plays a role in B cell activation.
- Though its exact function is not fully understood, IgD is believed to initiate early immune responses and the maturation of B cells.
Role of Antibodies in the Immune Response
Antibodies are central to the immune system’s ability to identify and eliminate pathogens. They participate in various immune mechanisms that neutralize or destroy foreign invaders.
- Neutralization:
- Antibodies can bind directly to pathogens (e.g., viruses and bacteria) or toxins, preventing them from entering host cells or exerting their harmful effects.
- Neutralizing antibodies can block viral entry into cells by binding to viral surface proteins or prevent bacterial adherence to tissues.
- Opsonization:
- Antibodies coat pathogens, marking them for ingestion and destruction by phagocytes, such as macrophages and neutrophils.
- The antibody’s Fc (stem) region interacts with Fc receptors on phagocytic cells, facilitating recognition and engulfment of the pathogen.
- Agglutination and Precipitation:
- Antibodies can cross-link multiple antigens, causing pathogens to clump together in agglutination. This makes it easier for immune cells to eliminate the pathogen in bulk.
- Precipitation occurs when antibodies bind to soluble antigens, forming complexes that are easier to clear from the bloodstream.
- Complement Activation:
- Certain antibodies, especially IgG and IgM, can activate the complement system, a group of proteins that work together to destroy pathogens.
- Complement activation leads to the formation of membrane attack complexes that can puncture bacterial cell membranes, causing cell lysis, or promote phagocytosis through opsonization.
- Antibody-Dependent Cellular Cytotoxicity (ADCC):
- In ADCC, antibodies bind to infected or abnormal cells (such as virus-infected or tumor cells), marking them for destruction.
- Natural killer (NK) cells recognize the antibody-coated cells through their Fc receptors and release cytotoxic molecules that kill the target cell.
- Immune Memory and Long-Term Immunity:
- After an infection, certain B cells become memory cells, retaining a memory of the specific antigen. Upon re-exposure to the same antigen, these memory B cells rapidly produce large amounts of antibodies, providing quicker and more effective protection.
- This ability of antibodies to provide immune memory is the foundation of long-term immunity and is exploited in vaccination.