Principle
-
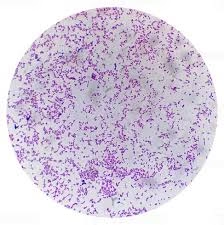
Gram Staining is a differential staining technique developed by Hans Christian Gram in 1884. It works on the basis of differences in the cell wall structure of bacteria. The fundamental principle of Gram staining is that the thick peptidoglycan layer in the cell wall of Gram-positive bacteria retains the primary stain (crystal violet), while Gram-negative bacteria, which have a thinner peptidoglycan layer and an additional outer membrane, do not retain the crystal violet and instead take up the counterstain (safranin).
-
Gram-positive bacteria:
-
Have a thick peptidoglycan layer in the cell wall.
-
Retain the crystal violet stain and appear purple/blue.
-
Examples include Staphylococcus aureus, Streptococcus pneumoniae.
-
-
Gram-negative bacteria:
-
Have a thin peptidoglycan layer and an outer membrane containing lipopolysaccharides.
-
Do not retain the crystal violet stain after decolorization and take up the safranin counterstain, appearing pink/red.
-
Examples include Escherichia coli, Salmonella typhi.
-
Materials Required
-
Glass Slides (clean and dry)
-
Bacterial Culture (broth or solid medium)
-
Crystal Violet Stain (Primary Stain)
-
Gram’s Iodine Solution (Mordant)
-
Ethanol or Acetone (Decolorizer)
-
Safranin Stain (Counterstain)
-
Distilled Water
-
Dropper or Pipette
-
Bunsen Burner (for heat fixation)
-
Coverslips
-
Microscope (with oil immersion objective)
-
Gloves and Safety Goggles
Procedure
Step 1: Preparation of Smear:
-
Place a clean glass slide on the staining rack.
-
For solid media: Take a loopful of bacterial culture from an isolated colony and place it in the center of the slide. Add a drop of distilled water and spread the bacteria with a sterile loop to make a thin smear.
-
For liquid culture: Place a drop of bacterial broth culture on the slide and spread it evenly with the sterile loop.
-
Allow the smear to air dry completely to avoid distortion of bacterial morphology.
-
Heat fixation: Gently pass the slide through the Bunsen burner flame 2-3 times to fix the bacteria onto the slide. This process kills the bacteria and ensures they adhere to the slide.
Step 2: Staining with Crystal Violet:
-
Flood the smear with crystal violet stain and leave it for 1 minute. Crystal violet is a primary stain that penetrates the bacterial cell wall and stains all bacteria initially.
-
Rinse the slide gently with distilled water to remove excess stain.
Step 3: Application of Gram’s Iodine (Mordant):
-
Flood the slide with Gram’s iodine solution and allow it to sit for 1 minute. Iodine acts as a mordant, forming a complex with crystal violet in the cell wall, which helps to enhance the retention of the crystal violet stain.
-
Rinse the slide with distilled water to remove excess iodine.
Step 4: Decolorization:
-
Apply ethanol or acetone (decolorizer) to the slide for 10-20 seconds. This step is crucial for the differentiation of Gram-positive and Gram-negative bacteria. The ethanol dissolves the outer membrane of Gram-negative bacteria and washes away the crystal violet, while Gram-positive bacteria retain the crystal violet-iodine complex due to their thicker peptidoglycan layer.
-
As soon as the runoff liquid becomes clear, rinse the slide with distilled water to halt the decolorization process.
Step 5: Counterstaining with Safranin:
-
Flood the slide with safranin stain and leave it for 30 seconds to 1 minute. Safranin is a red counterstain that stains Gram-negative bacteria, which have lost the crystal violet stain during decolorization.
-
Rinse the slide with distilled water to remove excess safranin and blot gently with a paper towel to dry.
Step 6: Microscopic Examination:
-
Place a drop of immersion oil on the smear.
-
Examine the slide under the 100x oil immersion objective lens of the microscope.
-
Begin with the 10x objective to locate areas of interest, and then use the 40x or 100x oil immersion objective to examine the detailed morphology of bacteria.
Observations
-
Gram-positive bacteria will appear purple/blue under the microscope due to the retention of crystal violet in the thick peptidoglycan layer.
-
Gram-negative bacteria will appear pink/red because they take up the counterstain (safranin) after the crystal violet is washed out.

Example:
-
Staphylococcus aureus (Gram-positive): Purple cocci clusters.
-
Escherichia coli (Gram-negative): Pink rods.
Precautions
-
Ensure the smear is thin to avoid clumping, which can interfere with bacterial identification.
-
Be cautious not to over-decolorize the slide, as this can cause Gram-positive bacteria to appear Gram-negative.
-
Always use fresh bacterial cultures, as older cultures may give unreliable results due to altered cell wall characteristics.
-
Handle staining reagents with care, wearing appropriate personal protective equipment such as gloves, goggles, and a lab coat.
-
Make sure to blot the slide gently to avoid damaging the bacterial smear.
-
Properly dispose of staining chemicals and used materials according to lab protocols.
References:
-
Collee, J.G., Fraser, A.G., Marmion, B.P. (2006). Mackie & McCartney Practical Medical Microbiology (14th ed.). Elsevier.
-
Horyn, J. et al. (2006). Gram Staining of Bacteria: Principles and Applications. Journal of Microbiology & Biology Education.
- Jorgensen, J.H., & Pfaller, M.A. (2015). Manual of Clinical Microbiology (11th ed.). American Society of Microbiology.
- Madigan, M.T., Martinko, J.M., & Bender, K.S. (2017). Brock Biology of Microorganisms (15th ed.). Pearson Education.