Introduction
- Intestinal nematodes (roundworms) are among the most common parasitic infections worldwide, affecting millions of people, particularly in tropical and subtropical regions.
- Their laboratory diagnosis involves a combination of methods to detect eggs, larvae, or adult worms in human samples, including stool, blood, or other bodily fluids.
- This detailed breakdown will delve deeper into the various laboratory diagnostic techniques for identifying intestinal nematodes.
Stool Examination
Stool examination is the primary and most widely used method for diagnosing intestinal nematode infections. It involves the direct microscopic examination of stool samples for the presence of eggs, larvae, or adult worms.
Steps in Stool Examination:
-
- Collection of Stool Samples:
- Multiple stool samples are often required because egg shedding can be intermittent.
- A minimum of three stool samples is usually collected over several days to maximize the chance of detecting parasitic forms.
- Fresh samples are preferred to avoid egg degradation.
- Direct Microscopic Examination:
- The stool sample is mixed with a small amount of saline (normal saline or iodine solution), and a smear is prepared on a microscope slide.
- The sample is then examined under a microscope (typically at 100x or 400x magnification) to identify eggs, larvae, or adult worms.
- Concentration Techniques: Concentration techniques enhance the ability to detect parasites by increasing the concentration of eggs, larvae, or adult forms. These methods include:
- Flotation Methods:
- A solution with a higher specific gravity than the eggs (e.g., zinc sulfate or sodium nitrate) floats the eggs to the surface. The eggs are then collected for microscopic examination.
- This method commonly detects Ascaris lumbricoides and Trichuris trichiura eggs, which float easily.
- Sedimentation Methods:
- Stool samples are mixed with water and allowed to settle (centrifugation may be applied) to concentrate heavier elements like hookworm eggs or larvae, which tend to sink to the bottom.
- The sediment is examined for the presence of eggs or larvae.
- Flotation Methods:
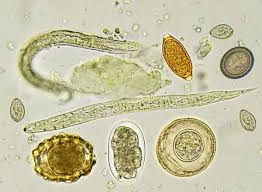
- Identification of Parasitic Forms: The different types of eggs, larvae, and adult forms have distinct characteristics, which allow for identification of the nematode species:
- Ascaris lumbricoides: Large, round eggs with a thick, mammillated (rough) outer shell.
- Enterobius vermicularis: Oval, flattened eggs with a distinctive asymmetry, usually found in the perianal region. The eggs are operculated (with a lid).
- Ancylostoma duodenale and Necator americanus: Transparent, thin-walled eggs containing an 8-cell stage.
- Trichuris trichiura: Barrel-shaped eggs with two polar plugs at either end, characteristic of whipworms.
- Strongyloides stercoralis: Larvae (rhabditiform or filariform) rather than eggs are commonly identified in stool samples.
- Collection of Stool Samples:
Stool Culture
Stool culture is particularly useful for detecting Strongyloides stercoralis, a nematode that can complete its life cycle inside and outside the human body.
Steps in Stool Culture:
-
- Preparation:
- A stool sample is incubated at room temperature for 48-72 hours.
- The incubation allows the larvae to mature from rhabditiform larvae (the diagnostic stage) to filariform larvae (the infective stage).
- Identification of Rhabditiform Larvae:
- After incubation, the sample is examined for rhabditiform larvae, characteristic of Strongyloides stercoralis.
- These larvae have a distinct bulbous esophagus and are recognized by size and shape.
- Limitations:
- This method is more time-consuming compared to direct stool examination.
- Not all laboratories routinely perform stool culture, requiring more specialized equipment and conditions.
- Preparation:
-
- Baermann Technique (For Hookworms)
The Baermann technique is a specialized method used to collect hookworm larvae from stool samples, particularly useful for diagnosing Ancylostoma and Necator species.
Steps in Baermann Technique
-
- Sample Collection:
- A small amount of stool is placed in gauze or mesh and submerged in warm water.
- Larval Migration:
- The larvae in the stool sample migrate out of the stool particles into the surrounding water.
- The water is left to stand for several hours, and the larvae can migrate into the liquid.
- Examination:
- After the larvae have migrated, the water is examined under a microscope for hookworm larvae.
- These larvae are rhabditiform and can be identified by size, shape, and characteristic structure.
- Sample Collection:
-
- Serological Tests
Serological tests detect specific antibodies or antigens the host produces in response to infection. These tests are particularly useful in cases where microscopic examination may not reveal the parasite or when the parasite is in a tissue phase.
Types of Serological Tests
-
- ELISA (Enzyme-Linked Immunosorbent Assay):
- ELISA can detect antibodies or antigens specific to intestinal nematodes like Strongyloides stercoralis and Trichinella spiralis.
- It is a highly sensitive test and can detect infections early, even before eggs are shed into the stool.
- Western Blot:
- This method involves separating parasite proteins using gel electrophoresis and transferring them to a membrane.
- Specific antibodies in the patient’s serum will bind to the proteins, and a reaction can confirm the diagnosis.
- Immunofluorescent Assay:
- This test uses antibodies labeled with a fluorescent dye to detect specific antigens or antibodies in the serum.
- It can detect Trichinella spiralis, Strongyloides stercoralis, and other nematodes.
- ELISA (Enzyme-Linked Immunosorbent Assay):
Molecular Techniques
Molecular techniques, particularly PCR (Polymerase Chain Reaction), are becoming more popular for diagnosing intestinal nematode infections due to their high sensitivity and specificity.
Application of PCR:
-
- Detection of Parasitic DNA:
- PCR amplifies specific regions of the nematode’s DNA found in stool, blood, or tissue samples.
- It can be used to detect Ascaris lumbricoides, Strongyloides stercoralis, and other intestinal nematodes.
- Advantages:
- PCR can identify the parasite even when eggs or larvae are absent from stool samples.
- It is especially helpful in diagnosing infections where adult worms are difficult to detect in fecal samples.
- Quantitative PCR:
- Quantitative PCR (qPCR) is a more advanced form of PCR that can quantify the amount of parasitic DNA present in a sample. It helps in assessing the severity of infection.
- Detection of Parasitic DNA:
Blood Tests
Blood tests can help diagnose intestinal nematode infections, particularly in cases where larvae are migrating through tissues, such as Strongyloides and Trichinella.
-
- Eosinophilia:
- Eosinophilia (an increased number of eosinophils in the blood) is a hallmark of parasitic infections, particularly in Strongyloides, hookworm, and Trichinella infections.
- Eosinophils are a type of white blood cell that are elevated in response to parasitic infections.
- The blood count can show marked eosinophilia during the larval migration phase or in cases of hyperinfection (especially in immunocompromised individuals).
- Larval Migration Indicators:
- In Strongyloides and hookworm infections, blood tests may show other signs of larval migration, such as elevated eosinophils, positive serology for specific antibodies, or larvae in blood smears.
- Eosinophilia:
Imaging Studies
In cases of hyperinfection or disseminated nematode infections (where larvae migrate to tissues or organs other than the intestines), imaging studies may be used to identify organ involvement.
Applications of Imaging:
-
- X-rays detect pulmonary involvement in cases of Strongyloides or hookworm infections. Löffler’s syndrome, associated with the migration of larvae through the lungs, can show up on chest X-rays as pulmonary infiltrates.
- CT Scan: Used for detecting more severe Trichinella spiralis infections, where the larvae can migrate to muscle tissues, leading to muscle inflammation or other organ damage.
- Ultrasound: Helps detect liver and abdominal involvement in cases of Trichinella or Strongyloides infections.