Introduction
- Iron deficiency anaemia occurs when the body’s iron stores are insufficient to meet the demands of red blood cell (RBC) production.
- Iron is a critical component of haemoglobin, the oxygen-carrying protein in RBCs.
- When dietary intake of iron is inadequate, or there is increased loss due to chronic bleeding (such as gastrointestinal ulcers or heavy menstruation), or increased requirement (such as during pregnancy or growth spurts), iron levels in the body begin to decline.
- Initially, the iron stored in ferritin is utilised to maintain erythropoiesis.
- As these stores become depleted, the synthesis of haemoglobin is impaired due to the lack of available iron.
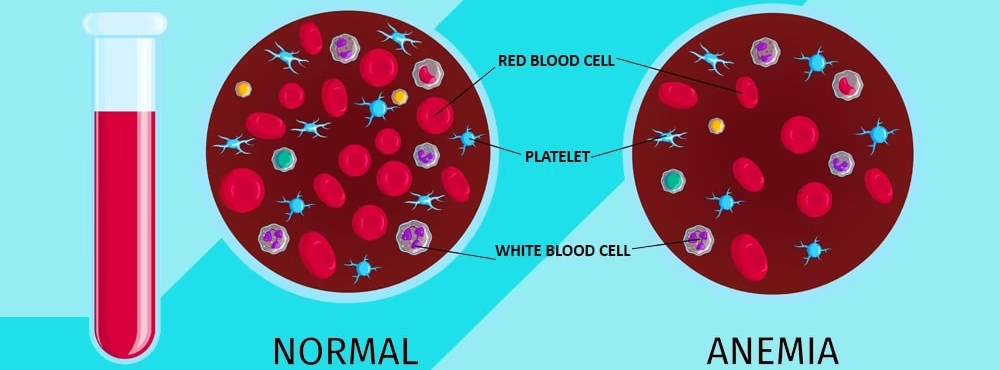
- This leads to the production of smaller (microcytic), paler (hypochromic) red blood cells with reduced oxygen-carrying capacity.
- Over time, the reduced haemoglobin concentration and defective erythropoiesis manifest as anaemia, characterised by fatigue, pallor, shortness of breath, and other systemic symptoms.
Causes
Iron deficiency anaemia can result from several factors, including:
- Insufficient dietary intake: Not consuming enough iron-rich foods, which is common in certain diets.
- Increased iron requirements: Periods like pregnancy, growth spurts, or breastfeeding increase iron needs.
- Blood loss: Chronic blood loss is a major cause, particularly from:
- Menstruation (especially heavy periods)
- Gastrointestinal bleeding (e.g., from ulcers, haemorrhoids, or conditions like inflammatory bowel disease)
- Poor iron absorption: Conditions that affect the digestive tract, such as celiac disease, certain surgeries, or gastrointestinal disorders, can interfere with iron absorption.
Symptoms
Common symptoms of iron deficiency anaemia include:
- Fatigue and weakness
- Pale skin
- Shortness of breath
- Dizziness or lightheadedness
- Brittle nails
- Unusual cravings (pica) for non-food substances (like ice or clay)
- Cold hands and feet
- Sore or swollen tongue
Laboratory investigations
- Laboratory investigations for iron deficiency anaemia focus on assessing iron levels, evaluating the degree of anaemia, and identifying any underlying causes.
- Iron deficiency anaemia is the most common type globally and is characterised by inadequate iron to support haemoglobin production, leading to decreased red blood cell production and oxygen-carrying capacity.
- Here’s an overview of the detailed laboratory workup for diagnosing iron deficiency anaemia.
Complete Blood Count (CBC)
The CBC provides a quantitative snapshot of the body’s hematologic status and is critical in detecting iron deficiency anaemia. Key components include:
- Haemoglobin (Hb):
- The iron-containing oxygen-transport protein in red blood cells (RBCs) is essential for oxygen delivery throughout the body.
- In iron deficiency anaemia, Hb levels drop because iron is necessary for haemoglobin synthesis.
- Typical haemoglobin cut-offs for diagnosing anaemia are <13 g/dL in men and <12 g/dL in women, though these values vary.
- Mean Corpuscular Volume (MCV):
- MCV measures the average size of RBCs.
- A low MCV (<80 fL) indicates microcytosis, characteristic of iron deficiency anaemia due to a lack of iron, resulting in smaller, poorly hemoglobinized RBCs.
- Early-stage iron deficiency, however, might show a normal MCV, known as “normocytic iron deficiency anemia,” which can progress to microcytic anaemia as iron depletion worsens.
- Mean Corpuscular Haemoglobin (MCH) and Mean Corpuscular Haemoglobin Concentration (MCHC):
- MCH and MCHC measure the amount of haemoglobin per RBC and the haemoglobin concentration within RBCs, respectively.
- Low MCH and MCHC are typical in iron deficiency, indicating hypochromia (pale cells), as each RBC contains less haemoglobin than normal.
- Red Cell Distribution Width (RDW):
- RDW quantifies the variation in RBC size.
- An elevated RDW reflects increased anisocytosis (variation in cell size), a hallmark of iron deficiency anaemia.
- The body produces smaller, iron-deficient RBCs, which coexist with any remaining normal-sized cells, thus increasing RDW.
Peripheral Blood Smear
The peripheral blood smear allows for a microscopic examination of RBC morphology, providing visual evidence of changes specific to iron deficiency:
- Microcytic, Hypochromic RBCs:
- RBCs appear smaller (microcytic) and pale (hypochromic) due to insufficient haemoglobin production, a key finding in iron deficiency anaemia.
- This finding results from the decreased availability of iron for haemoglobin synthesis, which produces cells with a low MCH and MCHC.

- Anisopoikilocytosis:
- Anisocytosis (variable RBC size) and poikilocytosis (abnormal RBC shape) are also frequently seen.
- Pencil cells (elongated RBCs) and target cells (RBCs with a bull’s-eye appearance) are common.
- Absence of Polychromasia:
- Polychromasia (immature, reticulated RBCs with residual RNA) is often absent, as iron deficiency limits the bone marrow’s capacity to produce new RBCs, reflecting ineffective erythropoiesis.
Reticulocyte Count
The reticulocyte count provides insight into the bone marrow’s response to anaemia:
- Low or Inappropriately Normal Reticulocyte Count:
- In iron deficiency anaemia, reticulocyte count is usually low or inadequately elevated for the degree of anaemia.
- Since iron is required for RBC production, the bone marrow cannot increase reticulocyte production in response to anemia.
- A higher reticulocyte count would be expected in cases where sufficient iron and nutrients allow the bone marrow to respond adequately.
Serum Ferritin
Ferritin serves as a marker of iron stores in the body and is often the most specific and sensitive indicator of iron deficiency:
- Low Serum Ferritin:
- Ferritin levels below 15-30 ng/mL are diagnostic of iron deficiency anemia in most cases, as they reflect depleted iron stores.
- Ferritin, an iron storage protein, is the first to decrease when iron stores are used up to compensate for deficient hemoglobin synthesis.
- Inflammation Limitation:
- Ferritin is also an acute-phase reactant that rises during inflammation or infection.
- In such cases, serum ferritin may be elevated even when iron stores are low.
- This limitation is particularly relevant when distinguishing between iron deficiency anemia and anemia of chronic disease (ACD).
Serum Iron, Total Iron-Binding Capacity (TIBC), and Transferrin Saturation
These iron panel components are crucial for evaluating iron’s transport and availability for hemoglobin production:
- Serum Iron:
- Measures the level of circulating iron bound to transferrin in the blood.
- In iron deficiency anemia, serum iron levels are low, as there’s insufficient iron available for erythropoiesis.
- Total Iron-Binding Capacity (TIBC):
- Reflects the amount of iron that transferrin (the iron transport protein) can bind.
- TIBC is often elevated in iron deficiency anemia because low iron levels trigger increased production of transferrin, increasing the body’s capacity to bind and transport iron in an attempt to capture any available iron.
- Transferrin Saturation:
- Transferrin saturation is calculated as (serum iron ÷ TIBC) × 100, showing the percentage of transferrin bound to iron.
- In iron deficiency anemia, transferrin saturation is typically low (<15%), as the body lacks enough iron to saturate transferrin.
Soluble Transferrin Receptor (sTfR)
Soluble transferrin receptor levels help differentiate iron deficiency anaemia from anemia of chronic disease:
- Elevated sTfR:
- In iron deficiency anaemia, cells increase the number of transferrin receptors on their surface to capture more iron.
- sTfR levels are, therefore, elevated.
- This test is especially useful when ferritin results are equivocal due to inflammatory conditions.
- Normal sTfR in Anemia of Chronic Disease (ACD):
- In ACD, iron stores are often adequate, but iron release is blocked by inflammation.
- sTfR remains normal in ACD, distinguishing it from iron deficiency anemia.
Red Cell Zinc Protoporphyrin (ZPP)
Red cell ZPP provides insight into iron availability during heme synthesis:
- Elevated ZPP:
- ZPP levels rise in iron deficiency anemia because zinc is incorporated into protoporphyrin in place of iron.
- Elevated ZPP indicates a disruption in heme synthesis, characteristic of iron deficiency, though ZPP can also be elevated in lead poisoning or chronic disease anemia.
C-reactive protein (CRP) or Erythrocyte Sedimentation Rate (ESR)
These inflammation markers aid in distinguishing iron deficiency anemia from anemia of chronic disease:
- Elevated CRP or ESR:
- CRP and ESR are elevated in inflammatory states, suggesting that ferritin results might be falsely high in inflammation.
- This distinction is crucial, as it helps clinicians interpret ferritin levels accurately and decide if additional tests are needed to confirm iron deficiency.
Bone Marrow
While not commonly required, a bone marrow biopsy can directly evaluate iron stores and confirm iron deficiency when other tests are inconclusive:
- Absence of Iron Stores: Staining for hemosiderin (an iron storage complex) in bone marrow macrophages confirms iron deficiency if no iron is detected. Bone marrow examination is generally reserved for complex cases where diagnostic uncertainty remains.
- Erythroid Hyperplasia: Increased production of erythroid precursors in the bone marrow is observed in response to anemia. However, these cells are microcytic and hypochromic, reflecting ineffective erythropoiesis in iron-deficient states.
Stool Occult Blood Test (FOBT) and Gastrointestinal Evaluation
Identifying chronic blood loss is especially important in adults with iron deficiency anemia, as GI bleeding is a common underlying cause:
- Positive Stool Occult Blood Test: A positive FOBT result suggests occult GI bleeding, which warrants further investigation, often through endoscopy or colonoscopy. Potential causes include peptic ulcers, polyps, or malignancies.
- Further Workup for Menstrual or Urinary Blood Loss: Excessive menstrual bleeding may account for iron deficiency in menstruating women. In some cases, urine analysis may be conducted to rule out blood loss from the urinary tract.
Urinalysis
Urinalysis can help identify hematuria (blood in urine), a less common source of chronic blood loss that could contribute to iron deficiency anemia:
- Microscopic Hematuria:
- Blood loss from the urinary tract may indicate underlying kidney or bladder conditions.
- Urinalysis helps detect hematuria, which may necessitate additional urologic investigations if iron deficiency anemia is suspected to arise from urinary blood loss.