Motility of Bacteria
- Motility refers to the ability of bacteria to move actively and independently.
- It is an important characteristic for identifying certain bacterial species and helps in distinguishing motile organisms from non-motile ones.
- Motility is often linked to the presence of flagella, pili, or other appendages that allow bacteria to move.
- The presence of motility in clinical samples is useful for identifying pathogenic bacteria in infections.
Methods for Determining Motility of Bacteria
Hanging Drop Method:
The hanging drop method allows the direct observation of bacterial motility in a liquid environment. It is a simple and effective way to observe the natural movement of bacteria under a microscope.
Procedure:
-
Place a small drop (about 1-2 µL) of bacterial culture in the center of a clean cover slip.
-
Carefully invert the cover slip and place it on the depression slide. The drop should hang freely in the depression.
-
Gently press the slide to create a seal and observe the drop under a microscope, using the 40x or 100x objective lens.
-
Observe the movement of bacteria in the drop. Motile bacteria will exhibit directional movement, while non-motile bacteria will remain stationary.
Advantages:
-
Provides a natural environment for observing motility, where bacteria move in their natural state.
-
It is quick and easy to perform.
-
Can differentiate between true motility and Brownian motion (random movement due to molecular collisions).
Disadvantages:
-
Requires careful handling to avoid drying of the drop.
-
Observation can be difficult if the concentration of bacteria is too high or too low.
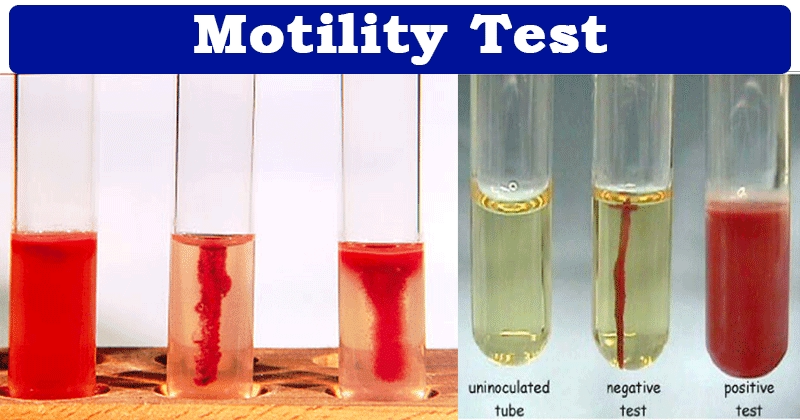
Semi-Solid Agar Method:
The semi-solid agar method is used to determine motility by inoculating bacteria into an agar medium that is less solid than typical agar. This allows motile bacteria to spread through the medium, whereas non-motile bacteria will only grow along the inoculation line.
Procedure:
-
Prepare a tube containing semi-solid agar (usually with 0.4% agar concentration).
-
Inoculate the center of the agar with a straight needle or a stab technique.
-
Incubate the culture at the appropriate temperature for a specified period (typically 24-48 hours).
-
After incubation, observe the pattern of growth. Motile bacteria will spread out from the point of inoculation, forming a diffused growth pattern. Non-motile bacteria will produce growth only along the inoculation line.
Advantages:
-
It is a straightforward and widely used technique.
-
Allows for easy differentiation between motile and non-motile bacteria.
Disadvantages:
-
Semi-solid media may not support the growth of some bacteria that require more solid conditions.
-
Less effective for bacteria that exhibit very weak motility.
Wet Mount (Direct Microscopic Examination):
The wet mount technique is used for the immediate observation of living bacteria in a liquid sample. This technique can be used to observe bacterial motility in clinical specimens, such as urine, sputum, or blood, to quickly detect motile organisms.
Procedure:
-
Place a small drop of bacterial culture on a clean glass slide.
-
Place a cover slip over the drop to avoid air bubbles and focus the sample under the microscope.
-
Observe the movement of bacteria under low to high magnification. Motile bacteria will move freely, whereas non-motile bacteria will remain stationary.
Advantages:
-
It is a fast and easy method to observe motility.
-
It allows for the direct observation of living bacteria in clinical samples, providing a clear view of their movement.
Disadvantages:
-
This method requires careful handling to prevent the sample from drying out.
-
Can be challenging to observe motility if the sample contains debris or too many bacteria.