Introduction
-
Nucleic acids are high–molecular-weight biological macromolecules that store, transmit, and express genetic information.
-
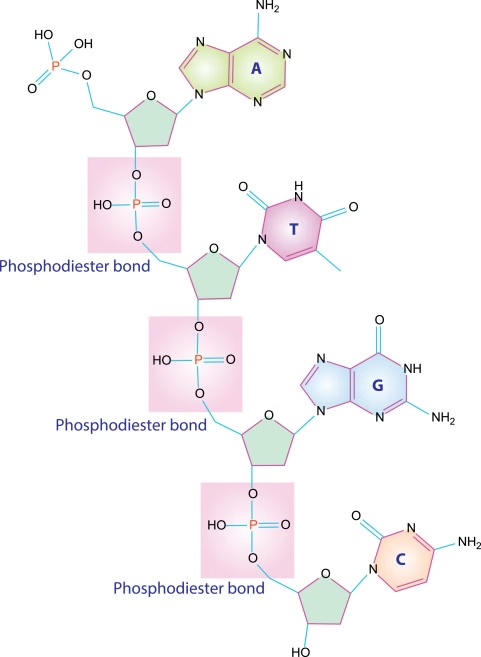
Chemically, they are polymers of nucleotides joined by 3′–5′ phosphodiester bonds.
-
Each nucleotide is composed of a nitrogenous base, pentose sugar, and phosphate group.
-
Two major types exist: DNA, the genetic material, and RNA, which mediates gene expression and protein synthesis.
-
Nucleic acids follow the central dogma of molecular biology: DNA → RNA → Protein.
-
They are essential for cellular function, heredity, and regulation of biological processes.
Historical Background
-
1869 – Friedrich Miescher first isolated nucleic acid (“nuclein”) from pus cells
-
1953 – Watson and Crick proposed the double-helical structure of DNA, establishing the molecular basis of heredity
-
Subsequent discoveries established the central dogma of molecular biology
Types of Nucleic Acids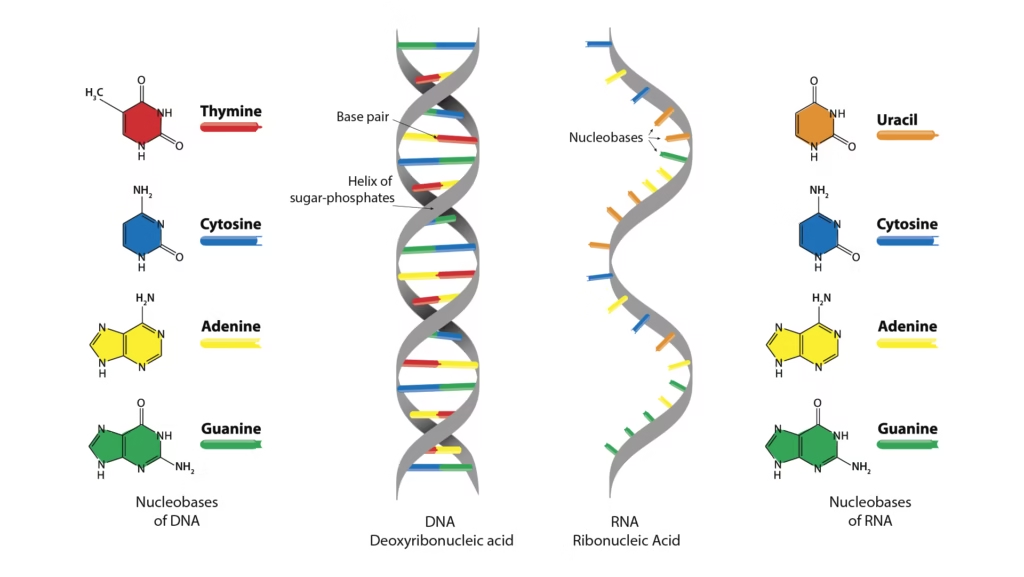
There are two principal types of nucleic acids:
-
Deoxyribonucleic Acid (DNA)
-
Primary genetic material in humans and most organisms
-
Located mainly in the nucleus, also in mitochondria
-
Chemically stable and self-replicating
-
-
Ribonucleic Acid (RNA)
-
Involved in expression of genetic information
-
Found in nucleus and cytoplasm
-
Chemically less stable, functionally diverse
-
Chemical Nature
-
Nucleic acids are polyanionic molecules due to phosphate groups
-
Contain:
-
Nitrogenous bases (purines and pyrimidines)
-
Pentose sugars (ribose or deoxyribose)
-
Phosphate backbone
-
-
Exhibit directionality: 5′ → 3′
Biological Importance
Nucleic acids are essential for:
-
Storage of genetic information (DNA)
-
Replication and inheritance
-
Protein synthesis (via RNA)
-
Cell differentiation and development
-
Regulation of gene expression
-
Evolution and mutation
Nucleotides
Nucleotides are low–molecular-weight organic compounds that serve as the basic structural units (monomers) of nucleic acids. They also perform independent and crucial metabolic functions beyond being building blocks of DNA and RNA.
Components of a Nucleotide
Each nucleotide is composed of three essential components:
A. Nitrogenous Base
-
Heterocyclic aromatic compounds containing nitrogen.
-
Two classes:
-
Purines (double-ring):
-
Adenine (A), Guanine (G)
-
-
Pyrimidines (single-ring):
-
Cytosine (C), Thymine (T), Uracil (U)
-
-
-
DNA bases: A, G, C, T
-
RNA bases: A, G, C, U
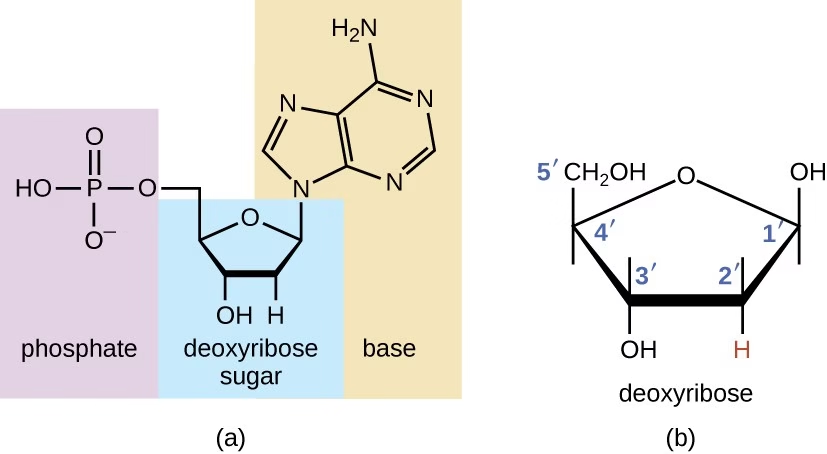
B. Pentose Sugar
-
A 5-carbon monosaccharide
-
Types:
-
β-D-2-deoxyribose → DNA
-
β-D-ribose → RNA
-
-
Difference:
-
Ribose has –OH at 2′ carbon
-
Deoxyribose has –H at 2′ carbon
-
-
Sugar carbons are numbered 1′ to 5′
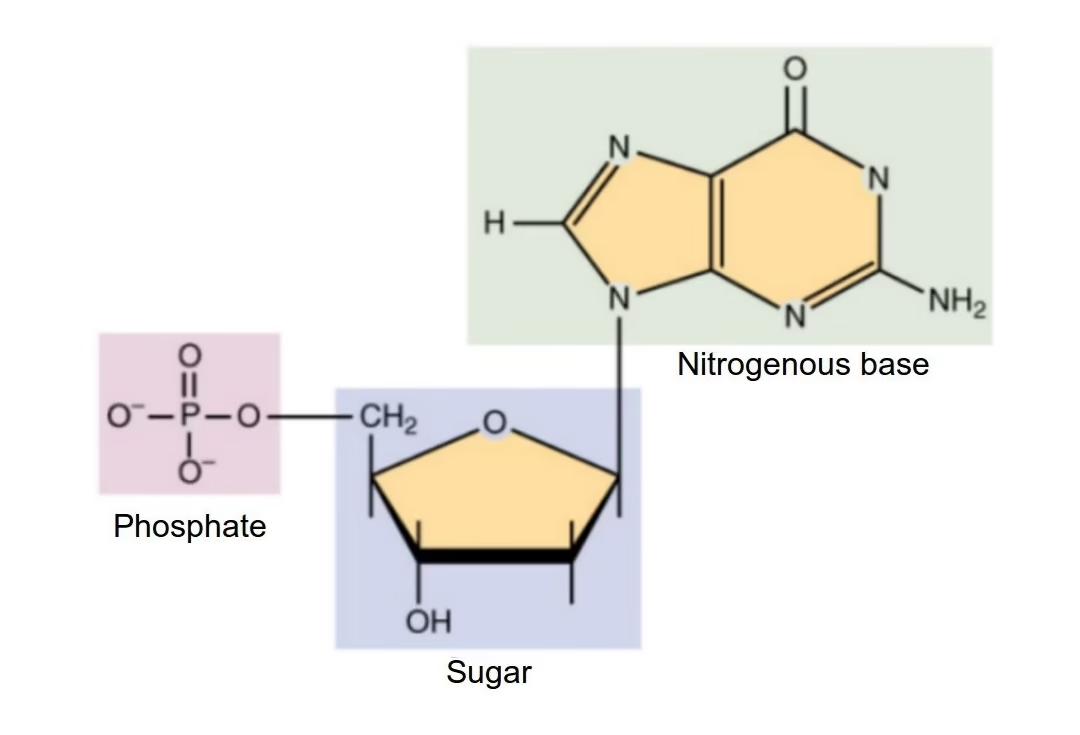
C. Phosphate Group
-
Derived from phosphoric acid
-
Attached mainly to the 5′ carbon of the sugar
-
One, two, or three phosphate groups may be present:
-
Monophosphate (AMP)
-
Diphosphate (ADP)
-
Triphosphate (ATP)
-
-
Responsible for:
-
Negative charge
-
Acidic nature
-
High energy bonds
-
Nucleoside vs Nucleotide
| Feature | Nucleoside | Nucleotide |
|---|---|---|
| Composition | Base + Sugar | Base + Sugar + Phosphate |
| Example | Adenosine | Adenosine monophosphate |
| Role | Intermediate | Functional & structural unit |
Types of Nucleotides
1. Based on the Type of Sugar
A. Ribonucleotides
- Contain β-D-ribose sugar
- Have –OH group at the 2′ carbon
- Found mainly in RNA
- Examples:
- AMP – Adenosine monophosphate
- GMP – Guanosine monophosphate
- CMP – Cytidine monophosphate
- UMP – Uridine monophosphate
Function: Protein synthesis, regulation, energy transfer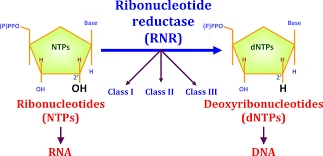
B. Deoxyribonucleotides
- Contain β-D-2-deoxyribose sugar
- Have –H at the 2′ carbon
- Found in DNA
- Examples:
- dAMP – Deoxyadenosine monophosphate
- dGMP – Deoxyguanosine monophosphate
- dCMP – Deoxycytidine monophosphate
- dTMP – Deoxythymidine monophosphate
Function: Storage of genetic information
2. Based on the Nitrogenous Base
A. Purine Nucleotides
- Contain purine bases (double-ring structure)
- Bases:
- Adenine (A)
- Guanine (G)
- Examples:
- AMP, GMP
- dAMP, dGMP
- Larger in size
Clinical relevance: Disorders lead to gout, immunodeficiency
B. Pyrimidine Nucleotides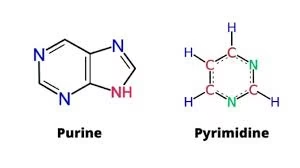
- Contain pyrimidine bases (single-ring structure)
- Bases:
- Cytosine (C)
- Thymine (T)
- Uracil (U)
- Examples:
- CMP, UMP, TMP
- dCMP, dTMP
Note: Thymine is present only in DNA; Uracil only in RNA
3. Based on the Number of Phosphate Groups
A. Monophosphates
- One phosphate group
- Examples:
- AMP, GMP, CMP
- Functions:
- Structural units of nucleic acids
B. Diphosphates
- Two phosphate groups
- Examples:
- ADP, GDP
- Functions:
- Energy transfer intermediates
C. Triphosphates
- Three phosphate groups
- Examples:
- ATP, GTP, CTP, UTP
- Functions:
- Energy currency
- Substrates for nucleic acid synthesis
High-energy bonds are present between phosphate groups
4. Based on Functional Role
A. Energy-rich Nucleotides
- ATP – universal energy currency
- GTP – protein synthesis, signal transduction
B. Coenzyme Nucleotides
- NAD⁺ / NADP⁺ – redox reactions
- FAD / FMN – electron transport
- Coenzyme A – acyl transfer
C. Second Messenger Nucleotides
- cAMP – hormone action
- cGMP – smooth muscle relaxation, vision
D. Activated Intermediate Nucleotides
- UDP-glucose – glycogen synthesis
- CDP-choline – phospholipid synthesis
5. Based on Cyclic Structure
Cyclic Nucleotides
- Phosphate forms a cyclic bond with sugar
- Examples:
- cAMP
- cGMP
- Act as intracellular second messengers
Formation of Polynucleotide Chain
- Nucleotides are linked by 3′–5′ phosphodiester bonds
- Bond formed between:
- 3′-OH of one nucleotide
- 5′-phosphate of the next nucleotide
- Results in:
- Sugar–phosphate backbone
- Directional strand (5′ → 3′)
Biologically Important Functions of Nucleotides
A. Structural Role
- Building blocks of DNA and RNA
B. Energy Currency
- ATP – universal energy carrier
- GTP – protein synthesis and signal transduction
C. Coenzymes
- NAD⁺ / NADP⁺ – oxidation–reduction reactions
- FAD / FMN – electron transport
- Coenzyme A – acyl group transfer
D. Second Messengers
- cAMP – hormone signaling
- cGMP – smooth muscle relaxation
E. Activated Intermediates
- UDP-glucose – glycogen synthesis
- CDP-choline – phospholipid synthesis
Acid–Base Properties
- Nucleotides behave as weak acids
- Phosphate groups ionize at physiological pH
- Contribute to:
- Buffering capacity
- Electrostatic interactions
Clinical Significance
- Defects in nucleotide metabolism cause:
- Gout (purine metabolism disorder)
- Severe combined immunodeficiency (SCID)
- Targets of many drugs:
- Anticancer agents
- Antiviral drugs
- Immunosuppressants
Structure of DNA
-
DNA (Deoxyribonucleic acid) is a double-stranded, helical nucleic acid that serves as the primary genetic material in humans and most organisms.
-
It is composed of deoxyribonucleotides linked by 3′–5′ phosphodiester bonds.
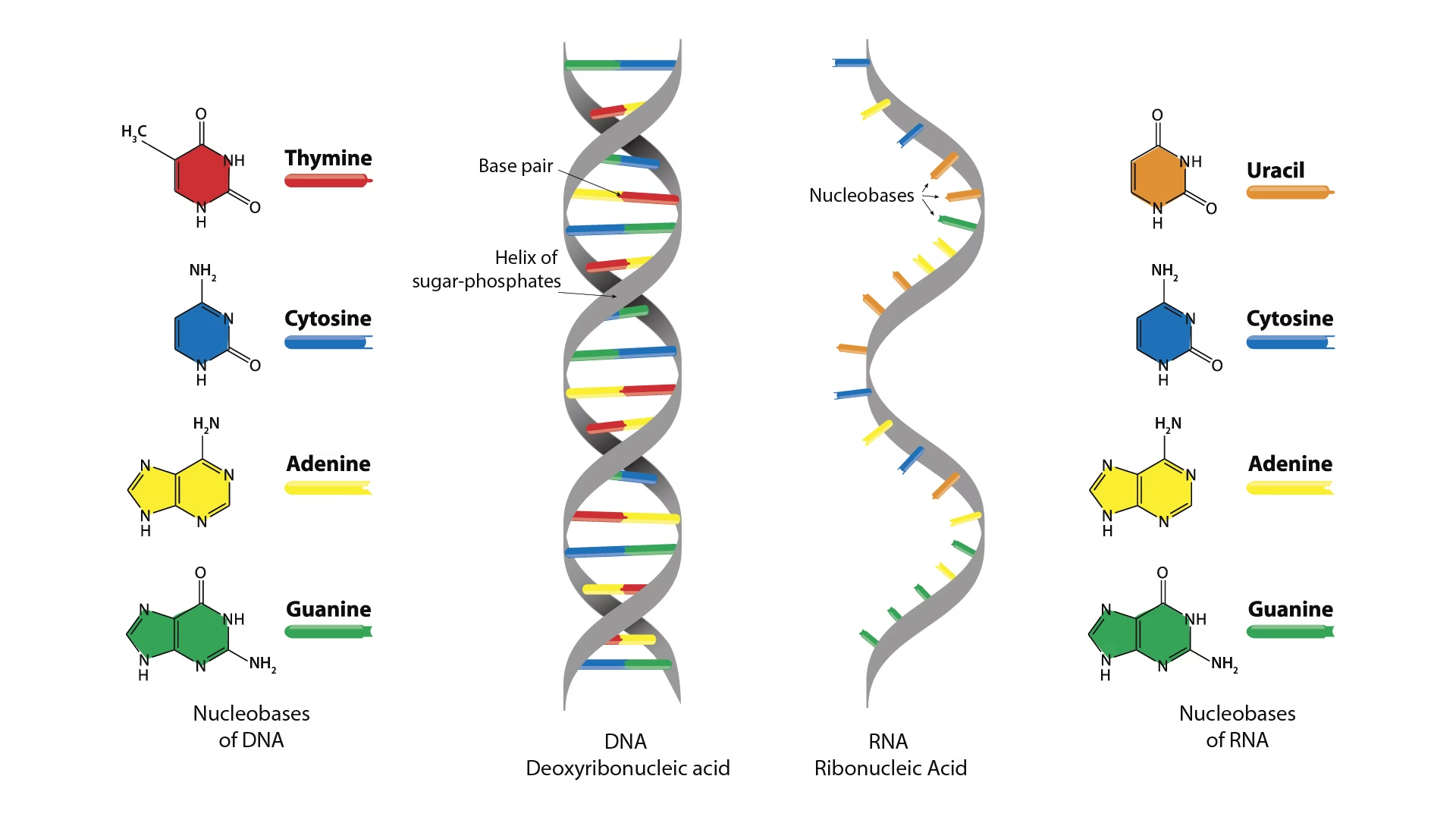
Watson–Crick Model (1953)
The accepted structural model of DNA proposed by Watson and Crick is based on X-ray diffraction data.
Structural Features
-
Double-stranded helix
-
Antiparallel strands
-
One strand runs 5′ → 3′
-
The other runs 3′ → 5′
-
-
Right-handed helix (B-DNA)
-
Sugar–phosphate backbone on the outside
-
Nitrogenous bases on the inside
Base Pairing
-
Complementary base pairing occurs via hydrogen bonds:
-
Adenine (A) pairs with Thymine (T) → 2 hydrogen bonds
-
Guanine (G) pairs with Cytosine (C) → 3 hydrogen bonds
-
-
This ensures the specificity and stability of the DNA molecule.
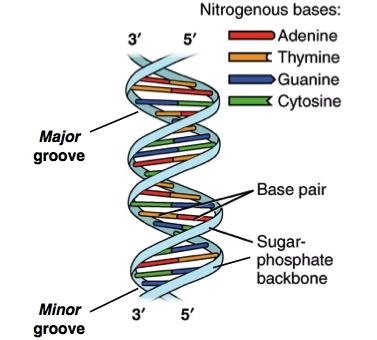
Dimensions of B-DNA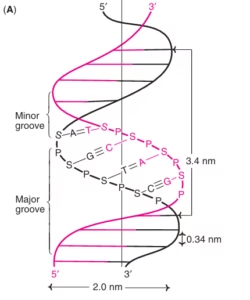
-
Diameter: 2 nm
-
Distance between base pairs: 0.34 nm
-
One complete turn: 3.4 nm
-
Approximately 10 base pairs per turn
Major and Minor Grooves
-
DNA helix has:
-
Major groove – wide, rich in genetic information
-
Minor groove – narrow
-
-
Important for:
-
Protein binding
-
Transcription factor recognition
-
Types of DNA
| Type | Characteristics |
|---|---|
| B-DNA | Most common, right-handed |
| A-DNA | Shorter, wider, dehydrated form |
| Z-DNA | Left-handed, GC-rich |
Function of DNA
1. Storage of Genetic Information
-
DNA stores hereditary information in the form of genes.
-
Information is encoded in the sequence of bases.
2. Self-Replication
-
DNA can replicate accurately during cell division.
-
Ensures transmission of genetic material to daughter cells.
3. Template for Transcription
-
DNA serves as a template for RNA synthesis.
-
Leads to formation of:
-
mRNA
-
tRNA
-
rRNA
-
4. Control of Protein Synthesis
-
DNA indirectly controls protein synthesis by:
-
Determining amino acid sequence
-
Regulating gene expression
-
5. Genetic Stability and Variation
-
Maintains genetic continuity
-
Mutations introduce genetic diversity
-
Basis of evolution and adaptation
6. Clinical and Biomedical Importance
-
DNA damage leads to:
-
Cancer
-
Genetic disorders
-
-
Basis of:
-
PCR
-
DNA fingerprinting
-
Gene therapy
-
Recombinant DNA technology
-
Organisation of DNA
-
In eukaryotic cells, DNA is highly organized and compacted to fit within the nucleus.
-
Approximately 2 meters of DNA is packed into a nucleus of about 10 µm diameter.
-
DNA associates with histone and non-histone proteins to form chromatin.
Chromatin
-
Chromatin is a nucleoprotein complex composed of:
-
DNA
-
Histone proteins
-
Non-histone proteins
-
-
Functions:
-
Packaging of DNA
-
Regulation of gene expression
-
Protection of genetic material
-
Histone Proteins
-
Small, basic proteins rich in lysine and arginine
-
Types:
-
Core histones: H2A, H2B, H3, H4
-
Linker histone: H1
-
-
A positive charge helps in binding negatively charged DNA
Nucleosome: Fundamental Unit of Chromatin
-
DNA is wrapped around a histone octamer:
-
2 × H2A
-
2 × H2B
-
2 × H3
-
2 × H4
-
-
147 base pairs of DNA wrap around the histone core
-
Linker DNA (20–80 bp) connects adjacent nucleosomes
-
Histone H1 stabilizes the nucleosome structure
Levels of DNA Packing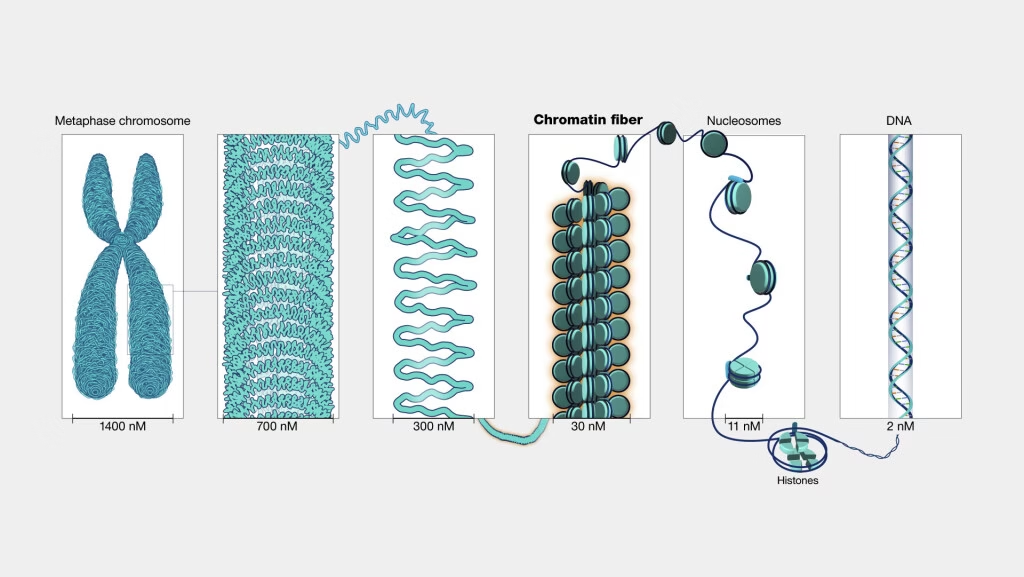
-
DNA double helix (2 nm)
-
Nucleosome fiber (10 nm) – “beads on a string”
-
30 nm fiber – solenoid or zig-zag model
-
Looped domains attached to scaffold proteins
-
Condensed chromatin
-
Metaphase chromosome
Euchromatin and Heterochromatin
A. Euchromatin
-
Lightly stained
-
Transcriptionally active
-
Gene-rich regions
-
Early replicating DNA
B. Heterochromatin
-
Darkly stained
-
Transcriptionally inactive
-
Gene-poor regions
-
Late replicating DNA
-
Types:
-
Constitutive (centromeres, telomeres)
-
Facultative (inactive X chromosome – Barr body)
-
Chromosomes
-
Highly condensed form of chromatin
-
Visible during cell division
-
Each chromosome has:
-
Centromere
-
Telomeres
-
p and q arms
-
Functional Significance of DNA Organisation
-
Efficient packaging of DNA
-
Protection from physical damage
-
Regulation of gene expression
-
Facilitates replication and transcription
-
Ensures proper chromosome segregation
RNA Structure and Function
-
RNA (Ribonucleic acid) is a single-stranded nucleic acid involved primarily in the expression of genetic information.
-
It acts as an essential intermediary between DNA and protein synthesis.
-
RNA is present in the nucleus, cytoplasm, and ribosomes.
Structure of RNA
1. Basic Components
RNA is a polymer of ribonucleotides, each consisting of:
-
Nitrogenous base
-
Ribose sugar
-
Phosphate group
2. Nitrogenous Bases
-
Purines: Adenine (A), Guanine (G)
-
Pyrimidines: Cytosine (C), Uracil (U)
-
Thymine is absent in RNA and replaced by uracil
3. Sugar
-
Contains β-D-ribose
-
Has a –OH group at the 2′ carbon, making RNA:
-
Less stable than DNA
-
More reactive chemically
-
4. Structural Features
-
Usually single-stranded
-
Exhibits secondary and tertiary structures due to:
-
Intra-strand base pairing
-
-
Has 5′ → 3′ polarity
-
Sugar-phosphate backbone with 3′–5′ phosphodiester bonds
Types of RNA and Their Functions
1. Messenger RNA (mRNA)
-
Carries genetic information from DNA to ribosomes
-
Contains codons (triplet nucleotide sequences)
-
Short-lived molecule
-
Template for protein synthesis
Function: Determines amino acid sequence of proteins
2. Transfer RNA (tRNA)
-
Smallest RNA molecule
-
Clover-leaf structure
-
Key structural parts:
-
Amino acid acceptor arm
-
Anticodon loop
-
D-loop and TΨC loop
-
-
Each tRNA is specific for one amino acid
Function: Transfers specific amino acids to ribosomes during translation
3. Ribosomal RNA (rRNA)
-
Most abundant RNA (~80%)
-
Combines with proteins to form ribosomes
-
Provides:
-
Structural framework
-
Catalytic activity (peptidyl transferase)
-
Function: Site of protein synthesis
Other functions of RNAs
| RNA Type | Function |
|---|---|
| snRNA | RNA splicing |
| snoRNA | rRNA modification |
| miRNA | Gene silencing |
| siRNA | RNA interference |
| lncRNA | Gene regulation |
Functions of RNA
-
Expression of genetic information
-
Protein synthesis
-
Gene regulation
-
Catalytic activity (ribozymes)
-
Development and differentiation
-
Defense mechanisms (RNA interference)
Comparisons: DNA vs RNA
| Feature | DNA | RNA |
|---|---|---|
| Strands | Double | Single |
| Sugar | Deoxyribose | Ribose |
| Base | Thymine present | Uracil present |
| Stability | More stable | Less stable |
| Function | Genetic storage | Gene expression |
MCQs
1. Nucleic acids are polymers of
A. Amino acids
B. Fatty acids
C. Nucleotides
D. Monosaccharides
✅ Answer: C
2. The basic structural unit of nucleic acids is
A. Base
B. Sugar
C. Phosphate
D. Nucleotide
✅ Answer: D
3. A nucleotide consists of
A. Base + sugar
B. Sugar + phosphate
C. Base + phosphate
D. Base + sugar + phosphate
✅ Answer: D
4. Which sugar is present in DNA?
A. Ribose
B. Fructose
C. Deoxyribose
D. Glucose
✅ Answer: C
5. Which sugar is present in RNA?
A. Deoxyribose
B. Ribose
C. Sucrose
D. Lactose
✅ Answer: B
6. Purine bases include
A. Cytosine and thymine
B. Uracil and cytosine
C. Adenine and guanine
D. Thymine and uracil
✅ Answer: C
7. Pyrimidine bases include
A. Adenine and guanine
B. Cytosine, thymine, uracil
C. Adenine and cytosine
D. Guanine and thymine
✅ Answer: B
8. Thymine is present in
A. RNA only
B. DNA only
C. Both DNA and RNA
D. Neither
✅ Answer: B
9. Uracil is present in
A. DNA only
B. RNA only
C. Both DNA and RNA
D. Neither
✅ Answer: B
10. The bond linking nucleotides is
A. Peptide bond
B. Glycosidic bond
C. Hydrogen bond
D. Phosphodiester bond
✅ Answer: D
11. Phosphodiester bond connects
A. 5′–5′ carbons
B. 3′–3′ carbons
C. 3′ carbon of one to 5′ carbon of another nucleotide
D. 2′–4′ carbons
✅ Answer: C
12. Direction of nucleic acid synthesis is
A. 3′ → 5′
B. 5′ → 3′
C. Random
D. Bidirectional
✅ Answer: B
13. The 5′ end of a nucleotide strand has
A. Free –OH group
B. Free base
C. Free phosphate group
D. Free sugar
✅ Answer: C
14. The 3′ end of a nucleotide strand has
A. Free phosphate
B. Free –OH group
C. Free base
D. Free nitrogen
✅ Answer: B
15. ATP is best described as
A. Structural protein
B. Energy currency of the cell
C. Hormone
D. Enzyme
✅ Answer: B
16. Which nucleotide acts as a second messenger?
A. ATP
B. GTP
C. cAMP
D. UTP
✅ Answer: C
17. NAD⁺ and FAD are
A. Hormones
B. Structural proteins
C. Coenzymes
D. Antibodies
✅ Answer: C
18. DNA is mainly located in
A. Cytoplasm
B. Ribosome
C. Nucleus
D. Golgi apparatus
✅ Answer: C
19. DNA structure was proposed by
A. Mendel
B. Franklin
C. Watson and Crick
D. Pauling
✅ Answer: C
20. DNA strands are
A. Parallel
B. Antiparallel
C. Circular
D. Random
✅ Answer: B
21. Base pairing in DNA is
A. A–C and G–T
B. A–U and G–C
C. A–T and G–C
D. A–G and T–C
✅ Answer: C
22. Number of hydrogen bonds between A and T
A. 1
B. 2
C. 3
D. 4
✅ Answer: B
23. Number of hydrogen bonds between G and C
A. 1
B. 2
C. 3
D. 4
✅ Answer: C
24. Most common form of DNA is
A. A-DNA
B. Z-DNA
C. B-DNA
D. C-DNA
✅ Answer: C
25. Major groove of DNA is important for
A. Replication only
B. Repair only
C. Protein binding
D. Mutation
✅ Answer: C
26. DNA packaging unit is
A. Chromosome
B. Histone
C. Nucleosome
D. Chromatid
✅ Answer: C
27. Nucleosome core contains
A. 6 histones
B. 8 histones
C. 10 histones
D. No histones
✅ Answer: B
28. Linker histone is
A. H2A
B. H2B
C. H3
D. H1
✅ Answer: D
29. Euchromatin is
A. Darkly stained
B. Condensed
C. Transcriptionally active
D. Inactive
✅ Answer: C
30. Heterochromatin is
A. Lightly stained
B. Transcriptionally inactive
C. Gene-rich
D. Early replicating
✅ Answer: B
31. RNA is generally
A. Double stranded
B. Single stranded
C. Circular
D. Triple stranded
✅ Answer: B
32. RNA contains which sugar?
A. Deoxyribose
B. Ribose
C. Glucose
D. Fructose
✅ Answer: B
33. RNA contains
A. Thymine
B. Uracil
C. Both
D. Neither
✅ Answer: B
34. Most abundant RNA is
A. mRNA
B. tRNA
C. rRNA
D. snRNA
✅ Answer: C
35. mRNA function is
A. Amino acid transport
B. Ribosome formation
C. Template for protein synthesis
D. Splicing
✅ Answer: C
36. tRNA function is
A. Gene regulation
B. Transport of amino acids
C. Ribosome formation
D. DNA synthesis
✅ Answer: B
37. Cloverleaf structure is seen in
A. mRNA
B. rRNA
C. tRNA
D. snRNA
✅ Answer: C
38. rRNA is involved in
A. Transcription
B. Replication
C. Protein synthesis
D. Splicing
✅ Answer: C
39. Central dogma of molecular biology is
A. RNA → DNA → Protein
B. Protein → RNA → DNA
C. DNA → RNA → Protein
D. DNA → Protein → RNA
✅ Answer: C
40. RNA is synthesized by
A. DNA polymerase
B. RNA ligase
C. RNA polymerase
D. Reverse transcriptase
✅ Answer: C
41. Which RNA is involved in gene silencing?
A. rRNA
B. tRNA
C. miRNA
D. hnRNA
✅ Answer: C
42. ATP contains
A. One phosphate
B. Two phosphates
C. Three phosphates
D. Four phosphates
✅ Answer: C
43. DNA replication is
A. Conservative
B. Dispersive
C. Semi-conservative
D. Random
✅ Answer: C
44. Nucleic acids are acidic because of
A. Sugar
B. Base
C. Phosphate group
D. Hydrogen bonds
✅ Answer: C
45. Which nucleotide is involved in glycogen synthesis?
A. ATP
B. GTP
C. UDP-glucose
D. cAMP
✅ Answer: C
46. Z-DNA is
A. Right-handed
B. Left-handed
C. Circular
D. Single stranded
✅ Answer: B
47. DNA backbone consists of
A. Base–base
B. Sugar–base
C. Sugar–phosphate
D. Phosphate–base
✅ Answer: C
48. Which nucleotide is used in protein synthesis?
A. ATP only
B. GTP
C. UTP
D. CTP
✅ Answer: B
49. RNA is less stable than DNA due to
A. Uracil
B. Single strand
C. 2′-OH group
D. Short length
✅ Answer: C
50. Genetic information is stored in
A. Proteins
B. Lipids
C. Carbohydrates
D. DNA
✅ Answer: D