Introduction
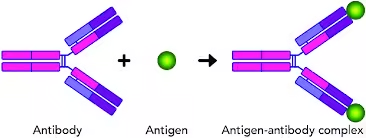
- Antigen-antibody reactions are the cornerstone of the immune system’s ability to recognize and neutralize foreign invaders.
- When an antigen (a foreign molecule or pathogen) enters the body, the immune system produces specific antibodies that bind to the antigen.
- This binding marks the pathogen for destruction or neutralizes its harmful effects.
- These highly specific reactions involve complex molecular interactions and are pivotal in immune defense mechanisms, diagnostic testing, and therapeutic interventions.
Salient Features of Antigen-Antibody Reactions
- Specificity:
- Each antibody is designed to bind to a unique antigenic epitope (the part of the antigen that the antibody recognizes). This specificity allows the immune system to differentiate between the body’s cells and foreign invaders.
- Specificity is crucial for precision in immune responses, reducing the likelihood of unintended damage to host cells and tissues.
- Reversibility:
- Antigen-antibody interactions are primarily non-covalent and reversible, relying on weak forces such as hydrogen bonds, van der Waals forces, and ionic bonds.
- This reversibility allows antibodies to form temporary complexes with antigens, an important aspect of immune regulation and pathogen clearance.
- High Sensitivity:
- The immune system can detect and respond to very low concentrations of antigens. This sensitivity is crucial for early pathogen detection and is leveraged in immunoassays and diagnostic tools.
- Multivalency:
- Both antibodies and antigens can have multiple binding sites. Multivalency strengthens the antigen-antibody interaction and enhances immune response by allowing multiple antibodies to bind simultaneously to a single antigen.
- Thermodynamic Considerations:
- Factors like temperature, pH, and ionic strength affect antigen-antibody interactions. For instance, high temperatures or extreme pH levels can destabilize the non-covalent bonds, potentially disrupting the interaction.
- Complement Activation:
- Some antibody classes (e.g., IgG, IgM) can activate the complement system when bound to antigens. This activation leads to immune responses, including cell lysis and inflammation, further aiding pathogen elimination.
Strength of Antigen-Antibody Reaction
- Affinity:
- Affinity is the binding strength between a single antigenic epitope and an antibody binding site. High-affinity interactions are stronger and more stable, making the antibody more effective in binding to and neutralizing the antigen.
- Factors influencing affinity include the antigen and antibody’s structural compatibility and the nature of the non-covalent forces.
- Avidity:
- Avidity refers to the overall strength of binding between a multivalent antigen (an antigen with multiple epitopes) and a multivalent antibody (an antibody with multiple binding sites).
- Avidity is typically greater than the affinity of individual interactions because multiple bonds stabilize the overall antigen-antibody complex, even if each binding is weaker.
- Avidity is particularly relevant in responses involving IgM antibodies, which have five binding sites, creating a stronger, more stable interaction with multivalent antigens.
Properties of Antigen-Antibody Reaction
- Non-Covalent Bonding:
- Non-covalent interactions, including hydrogen bonds, hydrophobic interactions, and ionic bonds, govern the antigen-antibody interaction. This type of bonding allows the interaction to be reversible and dynamic.
- Zone of Equivalence:
- The relative concentrations of antigens and antibodies influence antigen-antibody reactions. When the concentrations are balanced (in the zone of equivalence), optimal lattice formation occurs, leading to visible reactions like precipitation and agglutination.
- Agglutination and Precipitation:
- Agglutination occurs when antibodies bind to particulate antigens, causing visible clumping, commonly used in blood typing.
- Precipitation: Occurs when antibodies bind to soluble antigens and form an insoluble complex, often used in laboratory assays.
- Neutralization:
- Neutralization involves antibodies binding to a pathogen or toxin, thereby blocking its ability to interact with host cells. This mechanism is essential for neutralizing viruses and bacterial toxins.
- Complement Activation:
- Some antigen-antibody complexes activate the complement system, a protein series that assists in pathogen elimination by causing cell lysis, enhancing phagocytosis, and promoting inflammation.
- Cross-Reactivity:
- Sometimes, antibodies generated against one antigen can bind to similar epitopes on a different antigen, leading to cross-reactivity. This property is helpful in some diagnostic tests but can lead to autoimmunity if self-antigens are mistakenly targeted.
Types of Antigen-Antibody Reaction
Precipitation Reaction
Principle:
- The precipitation reaction occurs when soluble antigens (e.g., proteins, polysaccharides) interact with antibodies in a solution. Under optimal conditions, antigen-antibody complexes form large lattice structures that precipitate out of the solution.
- The formation of a precipitate is visible and depends on the relative concentrations of antigen and antibody. The reaction is most effective when the concentration of antigen and antibody is balanced, typically in the zone of equivalence. No precipitate forms if the antigen concentration is too high or low (the zone of antibody excess or antigen excess, respectively).
Types of Precipitation Reactions:
- Single Diffusion:
- Only one of the reactants (either antigen or antibody) diffuses through an agar medium to interact with the other. This method is mainly used for qualitative testing.
- Double Diffusion (Ouchterlony Test):
- Both the antigen and antibody diffuse from separate wells in an agar plate. The diffusion of both components allows them to meet at the zone of equivalence, where a precipitate forms. This test is often used for antigen characterization and comparing antigenic similarity.

- Both the antigen and antibody diffuse from separate wells in an agar plate. The diffusion of both components allows them to meet at the zone of equivalence, where a precipitate forms. This test is often used for antigen characterization and comparing antigenic similarity.
- Radial Immunodiffusion:
- Antibody is embedded in the agar, and antigen diffuses from a well. The resulting precipitate forms a ring around the well, with the size of the ring proportional to the amount of antigen.
Applications:
- Identification of Antigens and Antibodies: The Ouchterlony test identifies bacterial antigens or determines the degree of cross-reactivity between antigens.
- Detection of Fungal and Bacterial Infections: The VDRL (Venereal Disease Research Laboratory) test, which detects antibodies in the blood, is an example of precipitation used for syphilis diagnosis.
Agglutination Reaction
Principle:
- Agglutination reactions involve clumping particulate antigens (such as bacterial or red blood cells) when interacting with specific antibodies. This interaction leads to visible aggregates or clumps, easily observed under a microscope or with the naked eye.
- The key difference between agglutination and precipitation is that agglutination involves particulate antigens (e.g., cells or latex beads), whereas precipitation involves soluble antigens.
Types of Agglutination Reactions:
- Direct Agglutination:
- It occurs when the antigen (such as red blood cells or bacteria) is naturally part of a particulate form and reacts directly with specific antibodies, causing clumping. For example, in blood typing, specific antibodies interact with antigens on the surface of red blood cells, causing agglutination.
- Indirect (Passive) Agglutination:
- This method attaches soluble antigens or antibodies to a solid surface (e.g., latex beads). Agglutination occurs when the appropriate antigen or antibody is present in the sample. This method detects specific antibodies or antigens in the patient’s serum.
- Hemagglutination:
- This is a special type of agglutination in which antibodies bind to red blood cells. It’s commonly used in blood typing and in the detection of viral infections (e.g., determining influenza virus strain).
Applications:
- Blood Typing: Identifying blood types (A, B, AB, O) using specific antibodies that agglutinate corresponding red blood cell antigens.
- Bacterial Identification: Detecting bacterial antigens using specific antibodies (e.g., Salmonella in the Widal test).
- Pregnancy Tests: Detection of human chorionic gonadotropin (hCG) using antibodies to agglutinate antigen-coated particles in urine samples.
Advantages:
- Simple and rapid results.
- Cost-effective and relatively easy to perform.
Disadvantages:
- Sensitivity can be affected by the number of antigenic sites on the particles.
- False positives or negatives can occur if the test is not properly optimized.
Complement Fixation
Principle:
- Complement fixation is based on the ability of the complement system to be activated by antigen-antibody complexes. The classical complement pathway is triggered when antibodies bind to antigens, activating complement proteins.
- If an antigen-antibody complex forms during the test, the complement is consumed (fixed). If the complement is fixed, it will not be available to lyse indicator cells (e.g., red blood cells or sheep erythrocytes). If the complement is not fixed (i.e., no antigen-antibody complex), it will cause lysis of the indicator cells.
Method:
- The test typically includes the antigen, serum (with potential antibodies), complement proteins, and indicator cells. If the antibodies bind to the antigen, the complement is consumed and cannot lyse the red blood cells.
- A negative result (no antigen-antibody binding) causes complement activation, leading to cell lysis and a visible color change.
Applications:
- Detection of Antibodies: Commonly used to detect antibodies against viral infections (e.g., herpes, influenza).
- Diagnosis of Syphilis: Complement fixation is used in the diagnosis of syphilis (e.g., Wassermann test).
- Autoimmune Disorders: Identifying antibodies involved in diseases such as rheumatoid arthritis.
Advantages:
- Highly sensitive and specific.
- Suitable for detecting low levels of antibodies or antigens.
Disadvantages:
- More complex and time-consuming compared to agglutination or precipitation.
- Requires careful interpretation due to the involvement of multiple variables (complement activity, antigen-antibody reaction, etc.).
ELISA – Enzyme-Linked Immunosorbent Assay
Principle:
- ELISA is a method to detect the presence of specific antigens or antibodies by using an enzyme-conjugated with an antibody or antigen. The enzyme catalyzes a reaction with a substrate, resulting in a color change. The intensity of the color is proportional to the amount of the substance being tested.
Types of ELISA:
- Direct ELISA:
- The antigen is attached to the surface of a microplate well, and the enzyme-labeled antibody binds directly to the antigen. The substrate is added, and the enzyme activity produces a color change.
- Indirect ELISA:
- The antigen is immobilized on the microplate, and an unlabeled primary antibody can bind to it. A secondary antibody, conjugated with an enzyme, binds to the primary antibody. The substrate is then added, leading to a color change.
- Sandwich ELISA:
- The plate is coated with a capture antibody. The antigen in the sample binds to the capture antibody. A detection antibody conjugated with an enzyme binds to the antigen. The enzyme activity produces a signal proportional to the amount of antigen present.
- Competitive ELISA:
- The antigen in the sample competes with an enzyme-labeled antigen for binding to the antibody on the plate. The intensity of the signal is inversely proportional to the amount of antigen in the sample.
Applications:
- Disease Diagnosis: ELISA is widely used in detecting antibodies against viruses (e.g., HIV), bacteria (e.g., Helicobacter pylori), and autoimmune diseases (e.g., rheumatoid arthritis).
- Cancer Detection: ELISA is used to detect cancer biomarkers such as PSA (prostate-specific antigen) for prostate cancer.
- Pregnancy Tests: Detecting hCG levels in urine to confirm pregnancy.
Advantages:
- Highly sensitive and quantitative.
- It can be adapted to detect a wide variety of antigens and antibodies.
- High throughput; suitable for large sample sizes.
Disadvantages:
- Requires specialized equipment and reagents.
- Prone to false positives due to nonspecific binding if not optimized properly.
Immunofluorescence
Principle:
- Immunofluorescence is a technique that uses antibodies conjugated to a fluorescent dye. The complex fluoresces under ultraviolet (UV) light when the antibody binds to its specific antigen.
- The fluorescence can be observed under a fluorescence microscope, providing information about the presence and localization of specific antigens in cells or tissues.
Types of Immunofluorescence:
- Direct Immunofluorescence:
- The primary antibody is conjugated directly to the fluorescent dye. This method is simple and quicker but may be less sensitive.
- Indirect Immunofluorescence:
- The primary antibody binds to the antigen, and a secondary antibody, conjugated to a fluorescent dye, binds to the primary antibody. This method amplifies the signal and increases sensitivity.
Applications:
- Pathogen Detection: Identifying bacteria or viruses in clinical samples (e.g., detecting the influenza virus in respiratory samples).
- Cellular and Tissue Analysis: Used in research to study cellular processes, protein localization, or changes in gene expression.
- Autoimmune Disease Diagnosis: Detecting autoantibodies (e.g., anti-nuclear antibodies in lupus).
Advantages:
- Provides high spatial resolution.
- Allows for the study of protein localization in living cells or tissues.
Disadvantages:
- Requires specialized equipment (fluorescence microscope).
- Prone to nonspecific binding if antibodies are not optimized.
Applications of Antigen-Antibody Reaction
- Diagnostics:
- Immunoassays: Tests like ELISA and Western blotting are based on antigen-antibody reactions and detect specific antigens or antibodies in blood or tissue samples.
- Blood Typing: Agglutination reactions are used to determine blood groups (ABO and Rh), ensuring safe blood transfusions.
- Pregnancy Tests: Detect the presence of human chorionic gonadotropin (hCG), a hormone indicating pregnancy, through antigen-antibody binding.
- Therapeutics:
- Monoclonal Antibody Therapy: Engineered antibodies target specific antigens associated with diseases, such as cancer or autoimmune conditions, and are widely used in targeted therapies.
- Passive Immunization: Administered antibodies provide temporary immunity against specific pathogens, used in treatments for rabies, tetanus, and other infections.
- Vaccine Development:
- Vaccines stimulate antibody production by introducing a harmless form of the antigen. This primes the immune system for faster, more effective responses upon exposure to the actual pathogen.
- Research Applications:
- Flow Cytometry: Uses fluorescently labeled antibodies to detect and analyze cell populations based on surface markers, aiding in immunology and cancer research.
- Immunohistochemistry: This technique uses antigen-antibody reactions to detect specific proteins within tissue samples, allowing for visualization of cellular structures.
- Complement System Activation Assays:
- These assays evaluate the ability of antigen-antibody complexes to activate the complement pathway, which is essential in understanding immune responses and in diagnosing complement deficiencies.
- Allergy Testing:
- Allergy tests, such as skin prick tests, measure antigen-antibody reactions to detect specific IgE antibodies in response to allergens, helping to identify the substances causing allergic reactions.