Principle
- Staining techniques is to improve the visibility of intestinal parasites (ova, cysts, trophozoites) within stool samples through selective staining and preservation methods.
- These techniques enhance the differentiation between parasitic forms and other non-parasitic components in the stool, facilitating the accurate diagnosis of parasitic infections.
- Staining methods, such as iodine and Trichrome, provide distinct coloration that highlights the specific morphological features of parasites, aiding in their identification under the microscope.
Materials Required:
-
Stool sample (freshly collected and properly preserved)
-
Glass slides (clean and dry)
-
Cover slips
-
Microscope with 10x, 40x, and 100x oil immersion objectives
-
Staining reagents:
-
Normal saline
-
Lugol’s iodine solution
-
Formalin (for preserving stool samples)
-
Trichrome stain (for enhanced visualization of protozoa)
-
Distilled water
-
-
Dropper or pipette
-
Wooden stick or disposable inoculating loop for mixing
-
Petri dishes or small containers for stool sample preservation
-
Safety equipment (gloves, goggles, lab coat)
-
Pipette for transferring sample
Procedure:
Sample Collection:
-
Collect stool samples in a clean, dry container, ensuring no contamination from urine or other substances.
-
Stool samples should be processed as soon as possible, as prolonged storage may alter the morphology of parasites.
Initial Sample Examination:
-
Macroscopic Examination: Observe the stool sample for any visible signs of parasites (e.g., visible worms or abnormal appearance).
Preparation of Wet Mounts:
- Principle:
- The saline wet mount allows the visualization of living organisms, such as trophozoites of protozoa, in their natural motile state.
- It is based on the principle that the isotonic saline solution preserves the structural integrity of the cells while allowing motility of trophozoites, which is critical for identifying certain protozoan parasites (e.g., Giardia).
- This technique helps in detecting both cysts and trophozoites, which are the infective forms of many parasites.
-
Saline Wet Mount:
-
Take a small portion of the stool sample (about the size of a pea) and place it on a clean glass slide.
-
Add one drop of normal saline over the sample.
-
Use a wooden stick or loop to mix the stool with saline, breaking up any clumps and ensuring uniform dispersion.
-
Place a coverslip over the sample gently, avoiding air bubbles.
-
Examine the slide under the microscope starting with the 10x objective. Then, switch to 40x to closely observe the sample.
-
Look for the following:
-
Motile trophozoites (actively moving protozoa, e.g., Giardia lamblia).
-
Cysts of protozoa (e.g., Entamoeba histolytica cysts).
-
Ova of helminths (roundworms, hookworms, etc.).
-
Larvae or other developmental stages of helminths.
-
-
-
Iodine Wet Mount:
-
After observing the saline mount, prepare a second slide by placing a small amount of stool on a clean slide and adding a drop of Lugol’s iodine solution.
-
Place the coverslip gently over the iodine-stained sample.
-
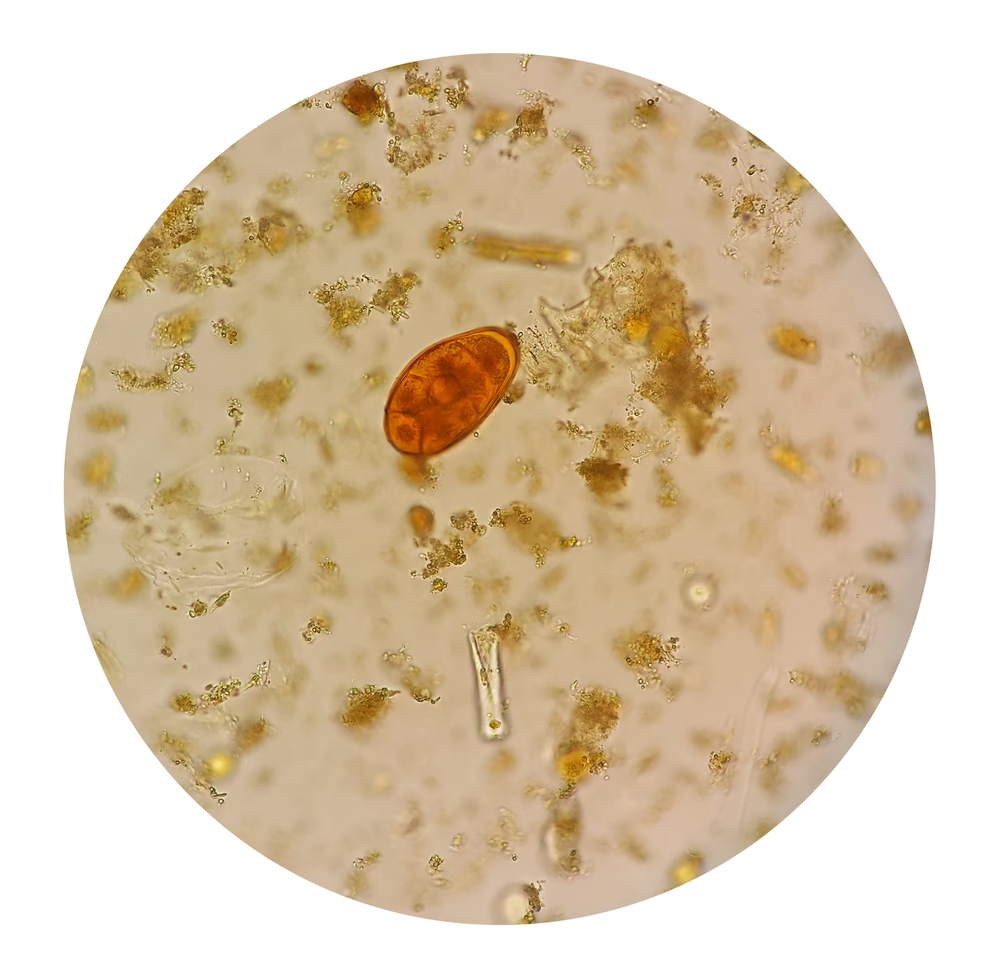
Examine under the microscope as described earlier, focusing on the iodine-enhanced clarity of cysts and ova. Lugol’s iodine helps stain the internal structures of cysts and makes helminth eggs more visible.
-
Protozoan cysts typically appear as clear, oval structures with internal details visible under iodine staining. Helminth eggs often show distinct outlines, and you may observe characteristics like yolk layers or embryonated forms.
-
Preservation of Stool Samples:
-
If immediate examination is not possible, preserve the stool sample by transferring it to a container with formalin (10% formalin is most commonly used). This will maintain the integrity of parasitic structures for future examination.
-
A small aliquot of the preserved stool can be sent to the laboratory for further processing or staining, such as Trichrome staining.
Trichrome Staining
- Principle:
- Trichrome stain is a multipurpose stain used to differentiate between various parasitic structures, particularly protozoan cysts and trophozoites.
- This technique uses a combination of dyes, typically green, blue, and red, to highlight the structures of protozoa, differentiating the cytoplasm, nuclei, and other internal organelles.
-
Trichrome Staining enhances the visual contrast and structure of protozoa and is useful for identifying protozoan trophozoites and cysts with more detail.
-
Procedure:
-
Fix the stool sample in formalin (if not already fixed).
-
Smear a thin layer of the preserved stool sample on a clean glass slide.
-
Air dry the slide and then fix it in absolute alcohol for 30 minutes.
-
Stain the slide with the Trichrome stain according to the manufacturer’s instructions. The stain will help differentiate protozoa from other material and make them easier to identify.
-
Rinse the slide with water and dehydrate using a series of alcohol solutions (70%, 95%, and absolute ethanol).
-
Mount the slide using a coverslip and examine under a microscope, typically at 40x and 100x magnifications.
-
-
Microscopic Examination:
-
Begin by focusing the slide with the 10x objective lens to locate the areas of interest.
-
Shift to the 40x objective for detailed examination.
-
If further clarity is required, use the 100x oil immersion lens to obtain high magnification.
-
Look for:
-
Protozoa cysts and trophozoites (e.g., Giardia, Entamoeba histolytica).
-
Helminth ova (e.g., Ascaris lumbricoides, Enterobius vermicularis).
-
Larvae or eggs with distinct characteristics:
-
Ova: These can be round or oval-shaped and may contain developing embryos. The eggs often have distinctive coverings.
-
Cysts: These are usually oval, containing one or more nuclei, and often surrounded by a membrane.
-
Trophozoites: Active motile stages of protozoa, which may exhibit flagella (e.g., Giardia) or amoeboid movement (e.g., Entamoeba histolytica).
-
-
-
Record the size, shape, and morphological features (e.g., number of nuclei in cysts, motility of trophozoites, presence of embryonated eggs) of the structures identified.
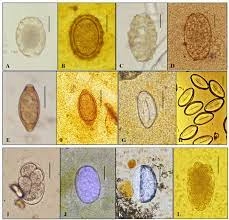
Identification and Diagnosis:
-
Parasites to be identified:
-
Giardia lamblia: Pear-shaped trophozoites with two nuclei and flagella.
-
Entamoeba histolytica: Cysts with 1–4 nuclei, chromatoid bodies, and cyst wall.
-
Ascaris lumbricoides: Ova with a thick, mammillated outer coat.
-
Hookworm: Oval-shaped eggs with a thin shell.
-
Strongyloides stercoralis: Larvae with a pointed tail.
-
Entamoeba coli: Larger cysts with up to 8 nuclei and a less distinct shape.
-
Recording Results:
-
Accurately record the findings in your practical report:
-
Include details such as the number of cysts/ova observed.
-
Note the morphological details (shape, size, internal structures).
-
Identify the species of parasites based on these observations and confirm using relevant diagnostic criteria.
-
Expected Results:
-
Saline Wet Mount: Presence of motile protozoa, cysts, and ova of helminths, depending on the parasitic infection.
-
Iodine Wet Mount: More detailed views of cysts and ova with enhanced clarity.
-
Trichrome Stain: Enhanced visualization of protozoan cysts and trophozoites, providing better diagnostic clarity.