
Introduction
- Semen analysis is a test performed to assess the quantity and quality of sperm within a male’s ejaculate.
- It’s an important diagnostic tool used to evaluate male fertility.
- This test is typically recommended when a couple has difficulty conceiving or as part of a general fertility workup.
- The semen analysis examines various aspects of the semen, including sperm concentration (count), motility (movement), morphology (shape), and additional parameters like pH, volume, and liquefaction time.
- The goal is to assess whether the sperm are healthy and capable of fertilizing an egg.
Production and Composition of Semen
Semen is a complex fluid produced by several male reproductive system parts. Its primary function is to transport sperm to the female reproductive tract during ejaculation. Let’s break down the key components:
Testicles (Testes) and Spermatogenesis:
- Location & Function: The testes are located in the scrotum and are responsible for producing sperm (spermatogenesis) and male sex hormones, especially testosterone.
- Spermatogenesis: This is the process by which sperm cells are produced. It occurs in the seminiferous tubules of the testes and takes about 64 to 72 days to complete. Immature sperm, called spermatids, eventually mature into sperm cells capable of fertilization.
- Testicular Dysfunction: Any impairment in testicular function, like low testosterone or varicocele (enlargement of veins in the scrotum), can reduce sperm count and quality.
Seminal Vesicles:
- Location & Function: The seminal vesicles are paired glands behind the bladder. They contribute about 60-70% of the total semen volume.
- Composition: They produce a fluid rich in fructose (which provides energy to sperm), prostaglandins (which help sperm motility and penetration), and proteins that protect sperm from the acidic environment of the female reproductive tract.
Prostate Gland:
- Location & Function: The prostate is a small gland just below the bladder, surrounding the urethra. It produces about 20-30% of the semen volume.
- Composition: The prostate secretes an alkaline fluid that helps neutralize the acidic environment of the vagina, allowing sperm to survive longer. This fluid contains zinc, citric acid, and enzymes that help activate sperm motility after ejaculation.
Bulbourethral Glands (Cowper’s Glands):
- Location & Function: These are small glands located at the base of the penis. They secrete a clear, slippery fluid during sexual arousal known as pre-ejaculate. This fluid helps neutralize any acidic residue in the urethra from urine and lubricates the urethra for sperm passage.
Sperm Cells:
- Components: Sperm consists of three parts:
- Head: Contains the nucleus with genetic material (DNA).
- Midpiece: Contains mitochondria, which provide energy for the sperm to move.
- Tail (Flagellum): Provides motility, enabling sperm to swim toward the egg.
Patient Instructions for Semen Collection
Correct semen collection is crucial for obtaining accurate results. Patients are typically given specific instructions to ensure the sample is good quality.
Abstinence:
- Why It’s Important: Abstaining from ejaculation for 2-5 days ensures optimal sperm count. If the man ejaculates too frequently (within 24-48 hours), the sperm count will be low. On the other hand, abstinence for too long can result in reduced sperm motility.
Collection Method:
- Procedure: The sample should be collected by masturbation into a sterile container. If a patient cannot provide a sample by masturbation, a special collection device (like a condom) may be used, but it should be free of any lubricants or chemicals that could damage sperm.
- Avoid Contamination: Collecting the entire sample is crucial, as losing even a small amount can significantly alter the results. Using any foreign object or lubricant can contaminate the sample and affect sperm health.
Transport and Handling:
- Temperature: Semen should be kept at body temperature (~37°C or 98.6°F) during transport to the laboratory. It should not be exposed to extreme temperatures, affecting sperm quality, especially motility.
- Timing: To preserve motility, the sample should be delivered to the laboratory as quickly as possible, ideally within 1 hour of collection.
- If the sample is delayed, sperm motility decreases, which could influence the analysis results.
Sample Container and Patient Sheet 
- Sterile Container: The semen sample must be collected in a sterile plastic container provided by the laboratory. This container is designed to prevent contamination and preserve the sperm for analysis.
- Patient Sheet: This document contains the instructions for proper sample collection, handling, and transport. It also outlines necessary information regarding abstinence, the procedure for providing the sample, and the importance of delivering the sample promptly to the lab.
Physical Examination of the Semen
The physical characteristics of the semen are critical indicators of reproductive health. A laboratory technician will assess the following parameters:
Volume:
- Normal Volume: The volume of semen typically ranges between 1.5 mL and 5 mL per ejaculation. Low volume (less than 1.5 mL) can indicate issues like a blockage in the seminal vesicles or prostate or low seminal fluid production.
- High Volume: Excessive volume could indicate problems like retrograde ejaculation, where semen flows backwards into the bladder.
Liquefaction Time:
- What is it?: Upon ejaculation, semen initially has a gel-like consistency, which helps sperm remain contained in the female reproductive tract. Within about 20 minutes, the semen should liquefy, allowing the sperm to move freely.
- Abnormal Liquefaction: If semen does not liquefy or takes longer than 60 minutes, it may suggest infection or prostate dysfunction. Infections like prostatitis can increase the production of proteins that delay liquefaction.
Colour:
- Normal Color: Semen is usually grayish-white.
- Yellow or Green Color: A yellowish colour may indicate urine contamination or infection (like a prostatic infection or seminal vesiculitis). A greenish hue might indicate bacterial infection.
- Clear or Watery: A watery appearance may indicate a low sperm count or seminal fluid production.
Appearance:
- Normal Appearance: The semen is generally opaque and slightly viscous. It’s important for sperm to be suspended in the fluid in a way that allows them to swim freely. If the semen is too watery, it could point to low sperm count or dilution due to excess fluid.
Viscosity:
- What is Viscosity?: Viscosity refers to the thickness or stickiness of the semen.
- Normal Viscosity: Normal semen should have a moderate viscosity. Too high or too low viscosity can impair sperm motility. High viscosity could indicate a blockage or infection, while very low viscosity may suggest a reduced sperm count or issues with fluid production.
pH:
- Normal pH Range: The pH of semen should be slightly alkaline, ranging between 7.2 to 8.0.
- Low pH: A pH below 7.2 suggests infection or a blockage in the male reproductive tract. Low pH can sometimes indicate asthenospermia (low sperm motility).
- High pH: A pH above 8.0 could indicate an issue with the prostate or seminal vesicles, potentially related to infection.
Motility:
It is a critical factor for fertility, as sperm need to swim through the cervix, uterus, and fallopian tubes to reach the egg.
-
- Progressive Motility: Sperm that moves forward in a straight line. This is considered the most important for fertilization.
- Non-progressive Motility: Sperm that move but not in a straight line or in circles. It’s less effective for fertilization.
- Immotile Sperm: Sperm that do not move at all.
-
Normal Motility: At least 40% of sperm should show progressive motility. Low motility may suggest infection, hormonal imbalance, or exposure to toxins (e.g., smoking, alcohol).
Chemical Examination of the Semen
pH Test:
Principle:
- Semen is naturally alkaline, with a typical pH range of 7.2–8.0. A healthy semen sample should fall within this range. The pH of semen is important because it helps neutralize the acidic environment of the female reproductive tract, allowing sperm to survive and swim towards the egg.
- A pH significantly outside this range may indicate infection, prostate problems, or other issues in the male reproductive system.
Reagents:
- pH Indicator Paper or pH Meter.
- pH Indicator Paper: It contains a special chemical dye that changes color in response to changes in the pH of the solution.
- pH Meter: This is a more precise tool that directly measures the pH value of the sample.
Procedure:
- Sample Collection: Collect semen into a sterile container, ideally after 2–7 days of sexual abstinence to ensure an accurate result.
- Indicator Paper Method:
- Dip a strip of pH paper directly into the semen sample.
- After a few seconds, remove the strip and compare its color to a standard pH chart.
- The color will match a corresponding pH value, indicating the semen’s pH.
- pH Meter Method:
- Calibrate the pH meter using buffer solutions (pH 4.0, 7.0, and 10.0).
- Immerse the pH electrode into the semen sample.
- Read the pH value directly from the meter display.
Interpretation:
- Normal pH: 7.2–8.0.
- A pH outside this range may indicate:
- Acidic pH (<7): Possible infection or prostate issues.
- Alkaline pH (>8.0): Can indicate infections, alkaline-producing conditions, or seminal vesicle dysfunction.
Fructose Test:
Principle:
- The seminal vesicles produce fructose, which is a vital energy source for sperm motility. It’s normally found in semen at a level of 200–500 mg/dL.
- A low fructose concentration could suggest a blockage in the seminal vesicles or dysfunction of these glands responsible for producing fructose.
Reagents:
- 1% Aqueous Iodine Solution: This reacts with fructose to form a coloured complex.
- Sodium Hydroxide (NaOH): Used to make the solution alkaline.
- Glacial Acetic Acid: Adjusts the pH and facilitates the reaction.
- Sulfuric Acid (H2SO4): Helps to complete the reaction.
Procedure:
- Sample Collection: Collect a fresh semen sample.
- Dilute the semen sample with distilled water (typically 1:1).
- Add 1–2 drops of iodine solution into the semen sample.
- Then add 3–4 drops of NaOH (strong alkaline solution).
- Add a few drops of glacial acetic acid and mix gently.
- Heat the sample in a boiling water bath for 5–10 minutes. This step helps to facilitate the reaction between iodine and fructose.
- After cooling, observe the solution.
- Positive Result: A blue color forms in the solution if fructose is present.
- Negative Result: No color change indicates that fructose is absent.
Interpretation:
- Normal Result: Fructose present in expected amounts (blue color forms).
- Low Fructose Levels: Suggest seminal vesicle dysfunction or obstruction. Could indicate conditions such as ejaculatory duct obstruction.
- No Fructose: May suggest congenital absence of seminal vesicles or a severe blockage.
Glucose Test:
Principle:
- Glucose in semen can be used as an alternative energy source for sperm. The test is used to measure the concentration of glucose, and abnormal levels might be linked to certain infections or metabolic disorders.
Reagents:
- Glucose Oxidase Reagent: Contains glucose oxidase, peroxidase, and a chromogenic substance (e.g., o-dianisidine), which reacts with glucose.
- Buffer Solution: Ensures the solution is at the correct pH for the test.
Procedure:
- Sample Preparation: Dilute the semen sample with saline (usually 1:1).
- Add the glucose oxidase reagent to the semen sample.
- Mix well and incubate the sample at room temperature for about 10–15 minutes.
- Observe any color change:
- Positive Result: A color change to blue indicates the presence of glucose.
- Negative Result: No color change means glucose is not detected.
Interpretation:
- Normal Result: Presence of glucose in semen in small amounts.
- High Glucose Levels: Can suggest the presence of an infection or certain metabolic disorders.
- No Glucose: Rare, but may indicate a problem with the seminal vesicles, which should normally secrete glucose.
Protein Test:
Principle:
- Semen contains proteins, particularly from the prostate and seminal vesicles. Protein levels are measured because they can provide information on prostate health, inflammation, or infection.
Reagents:
- Biuret Reagent: A solution containing sodium hydroxide (NaOH) and copper sulfate (CuSO₄), which reacts with peptide bonds in proteins.
- Sodium Chloride (NaCl): Used for dilution, if needed.
Procedure:
- Sample Preparation: If needed, dilute the semen sample with saline.
- Add the Biuret reagent to the semen sample in a test tube.
- Mix the solution thoroughly and incubate at room temperature for a short time (2–5 minutes).
- After incubation, observe the color of the mixture:
- Positive Result: A violet or purple color indicates the presence of proteins. This is due to the copper ions binding to peptide bonds in proteins.
- Negative Result: No color change means proteins are absent or present in very low amounts.
Interpretation:
- Normal Result: Proteins are present in the semen, typically in small amounts.
- High Protein Levels: Could indicate infection, inflammation, or prostate abnormalities.
- No Protein: This is rare but might indicate a problem with seminal fluid production.
Microscopic Examination of the Semen
Sperm Motility:
Principle:
- Sperm motility refers to the ability of sperm to move actively and efficiently. Sperm must swim through the female reproductive tract to reach and fertilize the egg. Motility is crucial for fertilization, and low motility can contribute to infertility.
- Motility is assessed by placing a drop of semen on a microscope slide and observing the sperm’s movement under a microscope.
Reagents/Equipment:
- Microscope: A standard light microscope is used, typically with a warm stage to maintain the semen sample at body temperature (37°C), as sperm motility is temperature-dependent.
- Cover Slip: A thin cover slip is placed on top of the sample to ensure proper focus and prevent evaporation of the semen.
Procedure:
- Sample Collection: Collect the semen sample into a sterile container. Having 2-7 days of abstinence before collecting the sample is ideal to ensure accurate results.
- Preparation: Place a small drop of semen onto the microscope slide and cover it with a cover slip.
- Examine Under Microscope: Using a light microscope (usually at 400x magnification), observe the sperm’s movement.
- Grading Motility: Motility is graded based on the speed and progression of the sperm:
- Grade A (Rapid progressive motility): Sperm move swiftly in a straight line or large, forward movements.
- Grade B (Slow progressive motility): Sperm move slowly, but still in a straight line.
- Grade C (Non-progressive motility): Sperm move but do not progress in a forward direction (e.g., circular or twitching).
- Grade D (Immotile): Sperm are not moving at all.
- Assess Motility (%): The percentage of motile sperm is determined, and the progressive motility is specifically calculated by observing sperm that move forward in a straight or curved line.
Interpretation of Results:
- Normal Motility: At least 40% of sperm should be motile, and at least 32% should exhibit progressive motility (WHO guidelines).
- Low Motility: If less than 40% of sperm are motile, it is considered asthenozoospermia (low sperm motility), which is often associated with infertility. Causes include infections, lifestyle factors (e.g., smoking, alcohol), or oxidative stress.
- Immotility: Complete immotility in the sperm (i.e., Grade D) could be a sign of severe sperm dysfunction or environmental factors (e.g., temperature changes or toxins).
Sperm Count (Concentration):
Principle:
- Sperm count refers to the number of sperm in a given volume of semen, typically expressed as millions of sperm per milliliter of semen. It helps to evaluate the male’s fertility potential.
- A high sperm count improves the chances of fertilization, while a low sperm count can indicate problems such as sperm production issues or blockages in the male reproductive tract.
Reagents/Equipment:
- Hemocytometer (or Neubauer Chamber): A specialized counting chamber used to count sperm in a controlled volume.
- Diluting Solution: Usually saline or a formalin solution, which is used to dilute the semen for counting. The dilution helps to make the sperm concentration low enough to count accurately.
- Microscope: Used for visualizing the sperm under the hemocytometer at 400x magnification.
Procedure:
- Sample Collection: Collect a fresh semen sample after 2-7 days of abstinence to ensure the most accurate result.
- Homogenize the Sample: Mix the sample gently to ensure uniform distribution of sperm.
- Dilution: Dilute the semen with a saline or formalin solution to reduce the sperm concentration, making it easier to count.
- Load the Hemocytometer: Place a drop of the diluted semen on the hemocytometer and cover it with a cover slip.
- Count Sperm: Under the microscope, count the sperm within the defined squares of the hemocytometer. Multiple squares are counted to calculate an average.
- Calculation: The sperm count is calculated by multiplying the number of sperm counted by the dilution factor and the volume of semen that corresponds to the number of sperm counted in the chamber.
Interpretation of Results:
- Normal Sperm Count: A normal sperm count is considered to be 15 million sperm per mL or more (according to WHO). The total sperm count in a typical ejaculate (1.5 to 5 mL) should be at least 39 million sperm.
- Low Sperm Count (Oligozoospermia): A count of fewer than 15 million sperm per mL is considered low sperm count, which may affect fertility.
- No Sperm (Azoospermia): If no sperm are present in the semen, it is referred to as azoospermia, which can be caused by obstructions, problems in sperm production, or conditions like testicular failure.
Sperm Morphology:
Principle:
- Sperm morphology refers to the size, shape, and structure of sperm. Proper morphology is crucial for the sperm’s ability to fertilize the egg. Abnormal sperm morphology can decrease the chances of successful fertilization, even if sperm count and motility are normal.
- The sperm head should be oval-shaped and smooth, the midpiece should be regular in shape, and the tail should be straight and uncoiled.
Reagents/Equipment:
- Papanicolaou Stain or Wright’s Stain: These are commonly used stains to highlight sperm structure under the microscope.
- Microscope: Typically used at 1000x magnification for evaluating sperm morphology at a cellular level.
Procedure:
- Sample Collection: Collect semen after 2-7 days of abstinence.
- Smear Preparation: Prepare a smear by placing a small drop of semen on a clean microscope slide.
- Staining: Stain the smear with Papanicolaou stain or Wright’s stain to enhance the visibility of sperm features.
- Examine Under Microscope: Using a microscope at 1000x magnification, examine the sperm to assess their shape.
- Morphological Classification:
- Head: Should be oval and smooth with a well-formed acrosome (the cap on the head that helps with egg penetration).
- Midpiece: Should be regular in size and shape. It contains mitochondria that provide energy for the sperm’s tail movement.
- Tail: The tail should be straight and long, with no kinks, coiling, or abnormalities.
Interpretation of Results:
- Normal Sperm Morphology: According to WHO guidelines, at least 4% of sperm should have a normal morphology.
- Abnormal Sperm Morphology: Abnormalities can include:
- Multiple Heads: Sperm with more than one head (due to defective sperm development).
- Tapered or Misshapen Heads: The head may be too round, too pointed, or misshapen, which impairs fertilization.
- Double Tail or Coiled Tail: Abnormal tail shapes that affect sperm motility.
- Headless or No Tail: These are usually immotile and incapable of fertilizing an egg.
Abnormal Sperm Identification: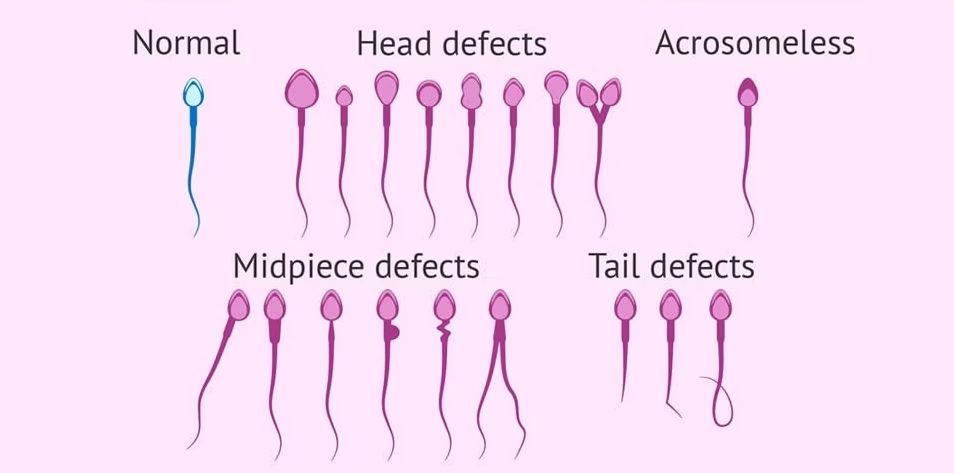
Types of Abnormal Sperm:
- Teratozoospermia: Refers to sperm with abnormal shapes. This is one of the most common findings in semen analysis and can include various defects such as:
- Multiple heads or double heads.
- Abnormally shaped heads or no heads at all.
- Coiled, bent, or double tails.
- Double midpieces or no midpieces.
- Asthenozoospermia: Refers to sperm that are poorly motile or not motile at all.
- Oligozoospermia: Refers to low sperm count.
- Azoospermia: The complete absence of sperm in the semen, indicating a serious issue with sperm production or an obstruction in the male reproductive system.
Microscopic Examination of Abnormal Sperm:
- Examine Sperm Shape: Look for defects in the head, midpiece, and tail. These defects can prevent sperm from reaching the egg or penetrating it effectively.
- Count the Percentage of Abnormal Sperm: If more than 50% of sperm are abnormal, it can significantly impact fertility. However, even if a high percentage of sperm are abnormal, assisted reproductive techniques like ICSI (intracytoplasmic sperm injection) can be used to overcome these issues.
Result Interpretation:
- High Abnormality Rate: A high rate of abnormal sperm (above 50%) may reduce fertility, as the sperm may be less capable of fertilizing an egg.
- Low Abnormality Rate: If less than 30% of sperm are abnormal, it might not significantly affect fertility, but it could still be a factor in cases of unexplained infertility.