Introduction
- The Single Radial Hemolysis (SRH) Assay is a serological technique for detecting virus-specific antibodies, particularly against hemolytic viruses.
- It is widely used in virology, immunology, and vaccine research to evaluate immune responses.
- This assay is based on antigen-antibody interactions, where antibodies in the test serum bind to a viral antigen embedded in an agarose gel containing complement and red blood cells (RBCs).
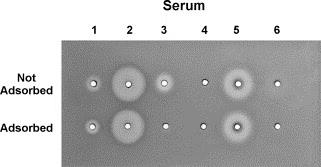
- If antibodies are present, complement activation leads to hemolysis (lysis of RBCs), creating a clear zone around the sample well.
- The size of the hemolytic zone is proportional to the antibody concentration, allowing for both qualitative and quantitative assessment of the immune response.
- SRH is commonly used for Influenza, Measles, and Rubella serology and is a reliable method for evaluating vaccine efficacy.
Principle
The Single Radial Hemolysis (SRH) Assay is based on three key mechanisms:
- Antigen-Antibody Binding – The viral antigen is incorporated into an agar gel. If specific antibodies are present in the serum sample, they bind to the antigen.
- Complement Activation – The presence of complement proteins in the gel leads to antibody-mediated activation of the complement cascade, which causes RBC lysis.
- Hemolysis Formation – Lysis of RBCs results in forming a clear zone around the well, indicating the presence and quantity of virus-specific antibodies.
Interpretations
- Larger Hemolysis Zone → Higher antibody concentration.
- Smaller or No Hemolysis Zone → Low or absent antibodies.
This principle makes SRH a sensitive and reliable method for assessing immunity after infection or vaccination.
Procedure
A. Preparation of Hemolysis Agar Plate
- Prepare agar medium containing:
- Buffered saline solution (PBS)
- Red Blood Cells (RBCs) (e.g., sheep or human RBCs)
- Viral antigen (e.g., Influenza, Measles, or Rubella antigens)
- Complement proteins (e.g., guinea pig complement)
- Pour the mixture into Petri dishes and allow the agar to solidify.
B. Sample Application and Incubation
- Punch small wells into the agar (approximately 3 mm in diameter).
- Add test serum samples (10-20 µL per well) into the wells.
- In a humid chamber, incubate the plates at 37°C for 18-24 hours.
C. Result Interpretation
- After incubation, observe the hemolysis zones under light.
- Measure the diameter of the clear zones using a ruler or caliper.
- Compare results with a standard antibody concentration curve for quantification.
Advantages of Single Radial Hemolysis
1. Rapid and Simple
- The assay provides quick results within 24 hours and does not require complex equipment.
- It is easy to perform and interpret, making it suitable for large-scale screening.
2. Quantitative and Qualitative
- The size of the hemolytic zone correlates with antibody concentration, allowing quantification of immune response.
- It helps in monitoring vaccination efficacy and natural infection immunity.
3. High Sensitivity and Specificity
- SRH is highly sensitive for detecting IgG antibodies against hemolytic viruses.
- It is less prone to non-specific reactions than other serological tests like ELISA.
4. No Need for Live Virus Handling
- Unlike viral culture methods, SRH does not require handling live infectious viruses, making it safer for laboratory personnel.
5. Useful for Vaccine and Immunity Studies
- SRH is widely used to evaluate vaccine-induced immunity, especially for Influenza and Rubella vaccines.
- It helps in determining seroprotection levels in populations.
Disadvantages of Single Radial Hemolysis
1. Requires Complement Proteins
- The assay depends on functional complement proteins, which must be carefully sourced and stored to prevent degradation.
2. Limited to Hemolytic Viruses
- Only useful for viruses that trigger hemolysis, such as Influenza, Measles, and Rubella.
- It cannot detect non-hemolytic viruses (e.g., HIV, Hepatitis B).
3. Requires Standardization
- The hemolysis zone measurement must be standardized, and variations in RBC concentration or antigen quality can affect results.
- Requires comparison with a standard antibody reference curve.
4. Possible False Negatives
- Low antibody titers may not generate detectable hemolysis, leading to false-negative results.
- Poor complement activity or degraded antigen may reduce test sensitivity.
5. Need for Skilled Interpretation
- Measuring hemolysis zones requires experience, and minor variations in incubation conditions may affect results.
Limitations of Single Radial Hemolysis
1. Not Suitable for All Viruses
- The SRH assay is restricted to viruses that cause complement-mediated hemolysis.
- For non-hemolytic viruses (e.g., Hepatitis B, Dengue, or SARS-CoV-2), ELISA or PCR-based tests are preferred.
2. Sensitivity Affected by Complement Source
- Complement proteins degrade over time, and their effectiveness may vary depending on the source and storage conditions.
3. Does Not Differentiate Antibody Types
- SRH primarily detects total antibodies (IgG, IgM, or IgA) but does not differentiate between recent infection (IgM) and past exposure (IgG).
4. Requires Fresh and Standardized RBCs
- RBC stability is crucial for accurate results, and variations in RBC quality can affect hemolysis zones.
- Freshly prepared standardized RBC suspensions are necessary for reproducibility.