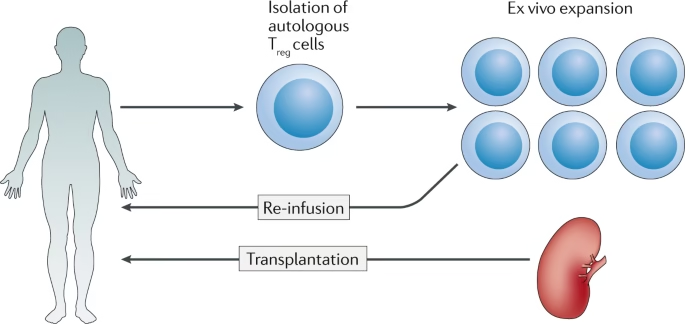
Introduction
- Tissue typing is a critical process in kidney transplantation that helps ensure compatibility between the donor’s and recipient’s tissues, minimizing the risk of rejection and improving the chances of long-term graft survival.
- Kidney transplants are performed when a patient’s kidneys are no longer functioning adequately, often due to chronic kidney disease (CKD) or kidney failure.
- Rejection—when the recipient’s immune system attacks the transplanted organ—remains one of the leading causes of graft loss, which is why tissue typing is essential for optimizing transplant outcomes.
- Tissue typing in kidney transplantation primarily involves determining the compatibility of Human Leukocyte Antigens (HLA) and blood types between the donor and recipient.
- Other factors, like crossmatch testing and panel reactive antibody (PRA) testing, are also vital in assessing immune compatibility.

Immune System and HLA
The immune system plays a central role in recognizing and defending the body from foreign substances, including transplanted organs. The Human Leukocyte Antigen (HLA) system is part of the Major Histocompatibility Complex (MHC), a gene group that regulates the immune response.
Components of the HLA System:
-
HLA Class I molecules (HLA-A, HLA-B, and HLA-C) are present on almost all nucleated cells. These molecules are important for presenting antigens to CD8+ cytotoxic T-cells, which can destroy cells that present foreign or abnormal proteins.
-
HLA Class II molecules (HLA-DR, HLA-DQ, and HLA-DP) are expressed primarily on antigen-presenting cells (APCs), such as macrophages, dendritic cells, and B-cells. These molecules present antigens to CD4+ helper T-cells, which coordinate the immune response, activating other immune cells to attack pathogens or foreign cells.
HLA Typing and Kidney Transplantation
HLA typing is identifying the specific HLA alleles (variations of HLA genes) present on both the donor’s and recipient’s cells. The closer the match of these HLA types between the donor and recipient, the more likely the transplant will be successful. Mismatches between HLA alleles can lead to immune rejection of the transplanted kidney.
Loci Involved in HLA Typing:
-
HLA-A, HLA-B (Class I MHC molecules): These are crucial in determining the compatibility between the donor and recipient’s cytotoxic T-cells. A mismatch can lead to acute cellular rejection.
-
HLA-DR (Class II MHC molecule): This is another key determinant of immune response. A mismatch at this locus may lead to chronic rejection, and it’s associated with the immune activation by helper T-cells.
Matching these loci between the donor and recipient is essential to reducing the risk of organ rejection and improving the kidney’s survival. Perfect matching, though ideal, is uncommon, especially with deceased donor transplants.
Types of HLA Matches
There are several degrees of HLA matching, based on the number of HLA loci that match between the donor and recipient:
-
Perfect Match: If the recipient and donor share identical alleles at all HLA loci (A, B, and DR), it is considered a perfect match. This reduces the risk of rejection and allows for a better long-term outcome.
-
Partial Match: If one or two HLA loci mismatch, the transplant can still be performed, but the risk of acute rejection is higher, and the patient may need more intensive immunosuppressive therapy.
-
Mismatched Transplant: The chances of rejection increase when there is a large mismatch in HLA alleles. However, transplantation can still occur with additional immunosuppressive measures or desensitization therapies.
Crossmatch Testing
Crossmatch testing is essential in assessing whether the recipient’s immune system will recognize the donor kidney as foreign. The test checks whether the recipient has pre-existing antibodies that would attack the donor organ immediately, resulting in hyperacute rejection.
How Crossmatch Testing Works:
-
The recipient’s serum (which contains antibodies) is mixed with the donor’s white blood cells (lymphocytes). If the recipient’s antibodies bind to the donor’s cells, the crossmatch is positive, indicating that the recipient’s immune system will likely attack the donor organ. This would prevent a successful transplant.
-
A negative crossmatch means the recipient’s serum does not have antibodies against the donor’s cells, making the transplant feasible with lower rejection risk.
Types of Crossmatch Tests:
-
CDC Crossmatch: In this traditional test, complement proteins destroy donor lymphocytes if antibodies in the recipient’s serum bind to the donor’s cells.
-
Flow Cytometry Crossmatch: This newer method is more sensitive and involves analyzing the interaction between the donor’s lymphocytes and the recipient’s antibodies using flow cytometry. It detects subtle antibody responses that may not be caught in CDC crossmatches.
Blood Type Compatibility
The ABO blood group system is another fundamental aspect of kidney transplant compatibility. Blood type compatibility is important because a mismatch can lead to hemolysis (destruction of red blood cells) and vascular rejection, resulting in immediate and severe complications.
Blood Type Compatibility:
- Type A: Can donate to Type A and Type AB recipients.
- Type B: Can donate to Type B and Type AB recipients.
- Type AB: Can receive kidneys from any blood type (universal recipient).
- Type O: Can donate to all blood types (universal donor), but can only receive a kidney from Type O donors.
ABO Incompatible Transplants:
- Sometimes, a kidney transplant may occur despite a blood type mismatch. Desensitization therapies, including plasmapheresis (to remove antibodies) and IV immunoglobulin (IVIg), reduce antibody levels and enable an ABO-incompatible transplant.
Panel Reactive Antibody (PRA) Testing
The Panel Reactive Antibody (PRA) test measures how sensitized the recipient is to foreign antigens, especially HLA antigens. It tests the recipient’s serum against a panel of donor cells to see if pre-existing antibodies could recognize and attack the transplant.
-
High PRA: A patient with a high PRA means they are highly sensitized and have antibodies against many HLA types. This makes finding a compatible donor more difficult and increases the risk of rejection.
-
Low PRA: A low PRA indicates that the recipient has fewer antibodies, meaning they are less sensitized and more likely to accept a transplant from a broader range of donors.
Immunosuppressive Therapy in Kidney Transplantation
Even with a perfect HLA match, immunosuppressive therapy is required to prevent the recipient’s immune system from attacking the transplanted kidney. These drugs suppress the immune system, reducing the likelihood of rejection while increasing the risk of infections and malignancies.
Common Immunosuppressive Medications:
-
Calcineurin Inhibitors (e.g., Tacrolimus, Cyclosporine): Block the activation of T-cells, the immune cells that typically reject the organ.
-
Corticosteroids (e.g., Prednisone): Reduce inflammation and overall immune system activity.
-
Antiproliferative Agents (e.g., Mycophenolate mofetil, Azathioprine): These medications inhibit the proliferation of T-cells and B-cells, key players in rejection.
-
mTOR Inhibitors (e.g., Sirolimus, Everolimus): Suppress T-cell activation and proliferation.
-
Monoclonal Antibodies (e.g., Basiliximab, Alemtuzumab): Target specific immune cells, such as T-cells, to prevent graft rejection.
Immunosuppressive therapy is carefully monitored to avoid complications like infection, cancer, and organ toxicity. The dosage of medications is adjusted based on the patient’s immune response, kidney function, and risk of rejection.
Desensitization Protocols
For patients who are highly sensitized (i.e., those with a high PRA) or ABO incompatible, desensitization protocols can be employed to reduce the antibodies that might attack the donor kidney. Desensitization therapies include:
-
Plasmapheresis: This procedure removes harmful antibodies from the recipient’s blood.
-
IV Immunoglobulin (IVIg): Helps modulate the immune system and reduce antibody levels.
-
Rituximab: An immunosuppressive drug used to deplete B-cells, which produce antibodies.
These therapies allow transplants to proceed even in difficult cases, such as when the recipient has a high PRA or ABO incompatibility.