Introduction
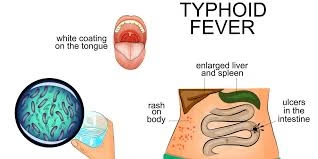
- Typhoid fever is caused by Salmonella enterica serotype Typhi (S. Typhi), a bacterium primarily affecting humans.
- It is transmitted through contaminated food and water, leading to severe systemic illness if not promptly treated.
- Despite the availability of vaccines and antibiotics, typhoid fever poses a significant health risk in many developing countries, necessitating effective diagnostic strategies.
Clinical Presentation
Symptoms
The symptoms of typhoid fever typically develop gradually and can vary in intensity. The following stages are commonly observed:
- Incubation Period: Ranges from 6 to 30 days, with most cases presenting symptoms within 1-2 weeks after exposure.
- Initial Symptoms:
- Gradual onset of fever (often continuous)
- Headaches
- Weakness and malaise
- Abdominal discomfort
- Progression:
- High fever (39-40°C or 102-104°F)
- Diarrhea (more common in the second week) or constipation
- Abdominal pain and tenderness
- Rash (rose spots) may appear on the abdomen or chest.
- Severe Complications:
- Intestinal perforation, leading to peritonitis
- Septicemia
Sample Collection
Specimen Types
Accurate specimen collection is essential for the laboratory diagnosis of typhoid fever:
- Blood Samples:
- Volume: A minimum of 10 mL per bottle is recommended for blood cultures to improve sensitivity.
- Timing: Blood samples should be collected during febrile episodes to maximize the likelihood of detecting the bacteria.
- Bone Marrow Aspirate:
- Particularly useful in cases with high suspicion of typhoid fever but negative blood cultures. This method enhances sensitivity.
- Stool Samples:
- Collected typically after the first week of illness when the likelihood of isolating S. Typhi increases. This can also help identify chronic carriers.
- Urine Samples:
- It may be useful in diagnosing chronic carriers or in later stages of the disease.
- Other Samples:
- In cases with severe complications, samples from the peritoneal cavity or other infected sites may be collected for analysis.
Laboratory Techniques
Blood Cultures
Culture Method
- Media: Blood is inoculated into enrichment broths (such as brain-heart infusion) and incubated under aerobic conditions. Blood culture media are often supplemented with nutrients and sodium polyanethole sulfonate (SPS) to inhibit phagocytosis.
- Incubation: Cultures are typically incubated at 35-37°C for 24 to 48 hours, with periodic shaking to enhance bacterial growth.
Identification
- Colony Morphology: If S. Typhi grows, colonies are usually small, smooth, and colorless on solid media like MacConkey or XLD agar.
- Biochemical Testing:
- Indole Test: S. Typhi is typically indole-negative.
- Lactose Fermentation: S. Typhi does not ferment lactose.
- Hydrogen Sulfide Production: Negative for hydrogen sulfide.
Bone Marrow Culture
- Bone marrow culture is performed by aspirating the bone marrow, usually from the iliac crest, and inoculating it into culture media.
- Sensitivity: It is more sensitive than blood culture, especially in patients who have received antibiotics before sampling.
Stool Culture
- Stool samples are analyzed for the presence of S. Typhi, especially in the second or third week of illness.
- This method is useful for identifying chronic carriers.
Serological Tests
Widal Test
- The Widal test detects antibodies against S. Typhi O (somatic) and H (flagellar) antigens.
- Procedure: Serum is diluted and mixed with specific antigens; agglutination indicates a positive reaction.
- Limitations:
- Low specificity and sensitivity, particularly in endemic areas where previous exposure may lead to false positives.
- The test is less reliable in the first week of illness and should not be used in isolation for diagnosis.
Molecular Methods
Nucleic Acid Amplification Tests (NAATs)
- Polymerase Chain Reaction (PCR): Detects S. Typhi DNA in clinical specimens (blood, stool, bone marrow).
- Advantages: High sensitivity and specificity, rapid results, and the ability to detect infections even after antibiotic treatment.
Real-Time PCR
- Allows for quantitative assessment and faster turnaround compared to traditional PCR.
Multiplex PCR
- This technique can detect multiple pathogens in a single assay, providing more comprehensive results.
Antigen Detection
- Commercial kits are available to detect S. Typhi antigens in stool or serum. These tests are not widely used but can provide rapid results.
Interpretation of Results
- Blood Cultures
- Positive Result: Isolation of S. Typhi confirms the diagnosis. It is most reliable during the first week of illness.
- Negative Result: A negative culture does not rule out typhoid fever, especially if the sample was taken after antibiotic initiation.
- Bone Marrow Culture
- Positive Result: More sensitive than blood culture, confirming the diagnosis, particularly in patients with persistent symptoms.
- Stool Culture
- Positive Result: Indicates active infection or chronic carrier status.
- Negative Result: This does not exclude the possibility of typhoid fever.
- Serological Tests
- Positive Widal Test: Indicates exposure to S. Typhi but must be interpreted cautiously due to potential cross-reactivity with other infections.
- Negative Widal Test: This does not exclude the diagnosis, especially in the early stages.
- Molecular Tests
- Positive PCR Result: Confirms the presence of S. Typhi, providing a reliable diagnosis.
- Negative PCR Result: This may occur if the bacterial load is too low or the test is conducted too late.
Clinical Implications
Treatment
- Antibiotics: Early and effective antibiotic treatment is crucial. Commonly used antibiotics include:
- Ciprofloxacin: First-line for adults, effective against most strains.
- Ceftriaxone: Used for severe cases or resistant strains.
- Azithromycin: An alternative in cases of resistance.
Supportive Care
- Fluid and electrolyte management is critical, especially in patients with diarrhea or dehydration.
Follow-Up
- Patients should be monitored for complications. Persistent symptoms may necessitate repeat testing or imaging studies.
Chronic Carriers
- Some individuals may become chronic carriers of S. Typhi, particularly after recovery. These individuals can shed the bacteria in their stools and may require treatment to eliminate the carrier state.
Public Health Considerations
Notification and Reporting
- Typhoid fever is a notifiable disease in many regions, requiring immediate reporting to public health authorities for outbreak control.
Vaccination
- Vaccination is recommended for individuals in endemic areas or those at high risk (e.g., travelers). Two types of vaccines are available:
- Vi polysaccharide vaccine: Effective for short-term protection.
- Typhoid conjugate vaccine: Provides longer-lasting immunity and is suitable for all age groups.
Preventive Measures
- Improving sanitation and access to clean water is crucial in controlling the spread of typhoid fever.
- Public health campaigns should promote hygiene practices and safe food preparation.
Challenges in Diagnosis
- Clinical Overlap: Symptoms can resemble those of other febrile illnesses (e.g., malaria, dengue), complicating the diagnosis.
- Access to Diagnostics: Limited access to laboratory testing in resource-limited settings can hinder timely diagnosis and treatment.
Advances in Typhoid Diagnostics
Emerging Technologies
- Rapid Diagnostic Tests (RDTs): New point-of-care tests are being developed to quickly detect S. Typhi antigens or DNA, facilitating early diagnosis in low-resource settings.
Genomic Studies
- Whole Genome Sequencing: Used for epidemiological surveillance, helping to track outbreaks and understand antibiotic resistance mechanisms.
Research Directions
- Investigating new vaccine candidates and improving existing vaccines are critical areas of ongoing research to enhance control measures against typhoid fever.