Introduction
- Using controls ensures histological staining results’ accuracy, consistency, and reliability.
- By using controls, histologists and pathologists can confirm that the staining reagents, techniques, and interpretation of stained tissue are accurate.
- Controls in various staining procedures help identify deviations, detect potential artefacts, and confirm the presence or absence of specific cellular components, pathogens, or other targets.
-
Hematoxylin and Eosin (H&E) Stain
- Positive Control: A known tissue section, such as a liver or kidney sample, is stained alongside test samples. This control ensures that hematoxylin binds effectively to nuclear material and eosin properly stains cytoplasmic and extracellular components.
- Application: If the nuclei in the control section appear distinctly blue or purple and the cytoplasm in pink, it confirms the staining process is functioning correctly.
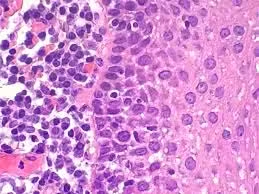
- Troubleshooting: If control nuclei are faint, it could indicate under-staining, pH issues with the hematoxylin, or degraded dye. If the eosin is not staining adequately, it may signal issues in dye concentration, pH adjustment, or tissue processing errors.
-
Special Stains
Special stains target specific tissue structures, cell components, or microorganisms, and each stain type has specific controls to confirm that it’s detecting its intended target.
Periodic Acid-Schiff (PAS) Stain
- Purpose: PAS stain highlights polysaccharides, mucins, and glycogen by oxidizing sugars and forming a magenta complex with Schiff reagent.
- Control Tissue: Known glycogen-rich tissue, like the liver or tissues with basement membrane components, are used as controls.
- Use of Controls:
- Positive Control: Shows a magenta colouration in regions containing glycogen or polysaccharides, confirming the PAS reagent and oxidation steps are working.
- Negative Control: This may involve diastase digestion in a second sample to remove glycogen and ensure that PAS staining is specific for carbohydrates other than glycogen.
- Troubleshooting: If the positive control fails to stain, it might indicate problems with oxidation or Schiff reagent quality. If the negative control still shows staining, it could suggest non-specific binding or contamination.
Congo Red Stain for Amyloid
- Purpose: Congo Red stains amyloid deposits, visible under polarized light as apple-green birefringence.
- Control: A positive control is a tissue section containing confirmed amyloid deposits, such as the spleen or liver.
- Outcome: Under polarized light, amyloid deposits should show green birefringence. Lack of birefringence in the control may signal improper staining, insufficient Congo Red application, or degradation of reagents.
Masson’s Trichrome Stain
- Purpose: Used for differentiating muscle (red) from collagen (blue or green) and cytoplasm (pink).
- Control Tissue: Sections with abundant muscle and collagen fibers, such as skeletal muscle or heart, serve as control tissues.
- Use of Controls:
- Positive Control: Shows clear muscle, collagen, and other components differentiation. If control tissue fails to differentiate well, this could indicate incorrect pH, dye concentration, or timing errors.
- Troubleshooting: If the control slide shows poor contrast between collagen and muscle, the acidic dye component (often aniline blue or light green) may need adjusting, or reagents may need to be freshened.
-
Microbial Stains
Microbial stains help identify bacteria, fungi, or acid-fast organisms. Controls are essential to confirm the stain’s specificity and effectiveness.
Gram Stain
- Purpose: Differentiates Gram-positive (purple) and Gram-negative (pink) bacteria.
- Controls:
- Positive Control Slide: A slide containing Gram-positive and Gram-negative bacteria, such as Staphylococcus (positive) and Escherichia coli (negative).
- Use of Controls:
- Positive Control: Gram-positive organisms should appear purple, and Gram-negative organisms should be pink. If both types stain the same colour, it could indicate decolourization issues or reagent errors.
- Troubleshooting: Inadequate decolorization may result in false Gram-positive results. Incorrect timing with safranin counterstain could also distort results.
Ziehl-Neelsen Acid-Fast Stain
- Purpose: Used to detect acid-fast organisms like Mycobacterium species.
- Control Slide: Contains known acid-fast bacteria (e.g., Mycobacterium tuberculosis).
- Outcome:
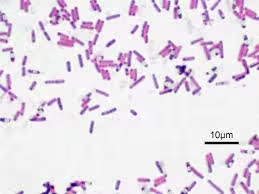
- Positive Control: Acid-fast bacteria should stain bright red against a blue background. If they do not appear, it suggests that the carbol fuchsin reagent or decolorizing solution may be ineffective.
- Troubleshooting: Failure of the acid-fast control indicates a need to check the strength and application of carbol fuchsin or the decolourization process with acid-alcohol.
-
Silver Stains
Silver stains are commonly used to identify fungi, reticular fibers, or certain bacteria. Silver staining is sensitive and requires proper control to avoid false positives or background staining.
- Gomori Methenamine Silver (GMS) Stain:
- Purpose: Highlights fungi and basement membranes.
- Control: A tissue sample known to contain fungal organisms or basement membranes is used as a positive control.
- Use of Controls:
- Positive Control: Shows fungi as black structures against a green or pale background. If fungi are not visible in the control, this could suggest silver solution preparation or timing issues.
- Troubleshooting: Brown or black background staining could indicate overexposure to silver, while no staining could indicate faulty silver solutions.
-
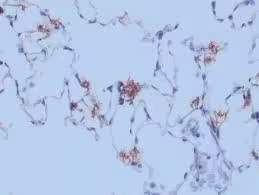
Immunohistochemistry (IHC) Controls
IHC stains are highly specific and use antibodies to detect antigens. Controls in IHC validate antibody specificity and staining accuracy.
- Positive Control: A tissue section known to express the target antigen ensures that the antibody binds correctly.
- Negative Control: A tissue lacking the antigen or processed without the primary antibody confirms the specificity of the antibody and prevents false positives.
- Outcome and Troubleshooting:
- Expected Result: The positive control should show appropriate staining, and the negative control should show no staining.
- If controls fail: Lack of staining in the positive control may suggest antibody degradation or incorrect preparation. Staining in the negative control suggests non-specific binding, indicating a need for blocking steps or antibody dilution adjustments.
-
In Situ Hybridization (ISH) Controls
- Purpose: ISH detects specific nucleic acid sequences within cells or tissues, allowing visualization of gene expression or viral DNA/RNA detection.
- Types of Controls:
- Positive Control: A tissue with a known target nucleic acid sequence presence ensures the probe works.
- Negative Control: A slide with a mismatched probe or non-complementary sequence helps confirm the specificity of the hybridization reaction.
- Outcome: Positive control tissues should show target staining, confirming the probe’s specificity. The negative control should show no staining, indicating no non-specific binding.
- Troubleshooting: Non-specific staining in the negative control suggests inadequate washing or overexposure. No staining in the positive control may indicate probe degradation or insufficient hybridization time.
Importance of Controls in Staining Procedures
Using controls across different staining methods provides multiple benefits:
- Quality Assurance: Controls validate staining quality and ensure reproducibility, allowing consistent results across different laboratories.
- Detection of Artifacts and Non-Specific Binding: Controls help identify background staining, non-specific antibody binding, or over/under-staining.
- Standardization and Compliance: Controls help establish baseline standards for diagnostic accuracy, ensuring that tissue staining meets clinical requirements.
- Troubleshooting and Optimization: Controls offer insight into potential problems with staining procedures, allowing technicians to fine-tune protocols and reagent concentrations.