Introduction
- The VDRL test was developed as a screening test for syphilis and is particularly used to detect secondary syphilis and the early stages of the disease.
- Syphilis progresses in phases: primary, secondary, latent, and tertiary.
- The VDRL test primarily detects antibodies produced by the body in response to the tissue damage caused by the Treponema pallidum bacteria.
- These antibodies are termed reagin antibodies, which are not specific to the bacterium but to certain lipids, primarily cardiolipin, released during infection.
- While the VDRL test is often used for screening purposes, it cannot provide a definitive diagnosis of syphilis.
- Treponemal tests, such as FTA-ABS (Fluorescent Treponemal Antibody Absorption) and TP-PA (Treponemal Pallidum Particle Agglutination), are necessary for confirmation.
Principle
The VDRL test operates on the flocculation principle, which involves the detection of reagin antibodies present in the patient’s serum. These antibodies react with cardiolipin-based antigens. The reaction produces visible clumping (or flocculation), indicating a positive result.
Steps involved in the reaction:
- Reagin antibodies, IgM and IgG types, are produced during infection due to tissue damage caused by Treponema pallidum.
- These antibodies react with lipid antigens (cardiolipin, lecithin, and cholesterol), which are components derived from beef heart or liver extracts.
- If reagin antibodies are present in the serum, the antigen will bind to them, causing flocculation (visible clumping).
- The presence of clumping is observed under direct light or using a microscope.
Requirements
- Sample:
- Serum: Most commonly used sample. Blood should be collected, allowed to clot, and serum separated by centrifugation.
- Cerebrospinal Fluid (CSF): Used in cases of suspected neurosyphilis or tertiary syphilis affecting the central nervous system.
- Reagents and Materials:
- VDRL Antigen: This consists of a cardiolipin-based antigen mixture. The antigen is prepared using extracts from beef heart or liver.
- Normal Saline (0.9% NaCl): Used to dilute the serum or to wash the RBCs if necessary.
- Control Sera: Positive and negative control sera verify the test.
- Positive Control: Serum is known to contain reagin antibodies.
- Negative Control: Serum from a healthy individual without syphilis.
- Glass Slides: To carry out the test (usually 3-4 slides for each sample and controls).
- Pipettes and Mixing Rods: For precise measurement and mixing.
- Incubator or Water Bath: Maintains incubation at a 37°C temperature during testing.
- Microscope (optional): For magnified examination of agglutination, particularly in quantitative testing.
Procedure
-
Qualitative Method (Screening Test)
- Preparation of Serum:
- Obtain blood and allow it to clot. After centrifugation, separate the serum.
- If the sample is too viscous, dilute it with normal saline (usually 1:1).
- Add Serum to the Slide:
- Place one drop (50 µL) of diluted serum onto a clean glass slide.
- Add VDRL Antigen:
- Place one drop (50 µL) of VDRL antigen onto the same spot.
- Mixing:
- Use a mixing stick or spatula to mix the serum and antigen gently. The antigen should be spread evenly over the serum on the slide.
- Incubation:
- Incubate the slide at 37°C for 8 minutes. Use an incubator or water bath to maintain the required temperature if needed.
- Observation:
- After incubation, examine the slide visually for flocculation (clumping).
- Positive result: The presence of clumps of antigen-antibody complexes.
- Negative result: No clumping.
- After incubation, examine the slide visually for flocculation (clumping).
-
Quantitative Method (Titration)
- Preparation of Serum Dilutions:
- Dilute the patient’s serum with normal saline in serial dilutions (e.g., 1:2, 1:4, 1:8, etc.).
- Add Antigen:
- Add VDRL antigen to each dilution.
- Incubation and Observation:
- Incubate as with the qualitative method. After incubation, observe the test tubes or slides for flocculation.
- Titer Determination:
- The highest dilution of serum at which visible flocculation occurs represents the titer of cold agglutinins in the serum. This can be used to gauge disease activity or monitor treatment progress.
Results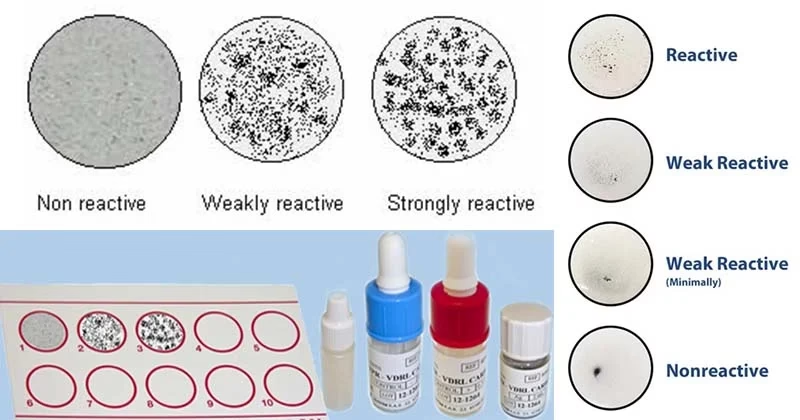
- Positive Result:
- The test is positive if visible clumping (flocculation) occurs at the test site. This indicates the presence of reagin antibodies in the serum.
- Strong agglutination indicates active or recent syphilis infection.
- Negative Result:
- No visible clumping or flocculation. The absence of agglutination suggests that the patient does not have syphilis or is in the tertiary latent stage where the antibody response is minimal.
Quantitative Results:
- The titer is the highest dilution of serum in which flocculation occurs. Higher titers suggest an active or severe infection, while lower titers indicate a previous or resolved infection.
Clinical Significance
The VDRL test is used to diagnose, monitor, and screen for syphilis, but it is not definitive. The test has several clinical uses:
- Screening for Syphilis:
- The VDRL test is part of routine screening for syphilis, especially in pregnant women (to prevent congenital syphilis) and individuals at high risk (those with multiple sexual partners, history of STIs, etc.).
- Diagnosing Syphilis:
- It is used to identify active syphilis (especially during primary and secondary syphilis stages), where the presence of reagin antibodies is typically high.
- It can also help in the diagnosis of neurosyphilis (if CSF is used).
- Monitoring Disease Progression:
- The test is often used to monitor treatment. The titer should decrease if effective treatment indicates a reduction in antibodies.
- A fall in titer suggests that the infection is under control, whereas a rise in titer could indicate reinfection or inadequate treatment.
- Non-Specific for Syphilis:
- The VDRL test is non-treponemal and cannot specifically confirm syphilis. A positive result is often confirmed with a treponemal test (such as FTA-ABS or TP-PA) to verify the presence of Treponema pallidum.
- Screening in High-Risk Populations:
- The test is frequently used to screen individuals in high-risk groups or those with symptoms of syphilis (like sores, rash, or neurological symptoms).
- It’s also used in some regions’ pre-employment or pre-marriage screening programs.
Limitations
- False Positive Results:
- Autoimmune diseases (e.g., lupus), pregnancy, malaria, leprosy, and viral infections can lead to false-positive results in the VDRL test.
- A positive VDRL test should be confirmed with a treponemal test for syphilis diagnosis.
- False Negative Results:
- The VDRL test may yield false-negative results in early syphilis (particularly during the primary stage) or in latent syphilis where antibody levels are low.
- The VDRL test is less sensitive in tertiary syphilis or late latent syphilis.
- Sensitivity and Specificity:
- The sensitivity of the VDRL test may vary depending on the stage of syphilis. It is most sensitive during secondary syphilis and less so in latent or tertiary syphilis.
- Its specificity is lower than treponemal tests, so it should be used with confirmatory tests.
Precautions
- Proper Storage of Reagents:
- VDRL antigen must be stored at the appropriate temperature (usually 2-8°C) to maintain its reactivity and avoid degradation.
- Correct Sample Handling:
- Ensure proper collection and storage of serum to avoid degradation of antibodies or antigen contamination.
- Temperature Control:
- Maintain the temperature at 37°C during incubation to ensure accurate results.