
Introduction
-
A spectrophotometer is an instrument used to measure how much light a substance absorbs at a particular wavelength.
-
It helps in determining the concentration of substances in solution based on the intensity of light absorbed.
-
The instrument works on the principle that every substance absorbs or transmits light over a specific range of wavelengths.
-
It follows Beer–Lambert’s law, which states that absorbance is directly proportional to the concentration of the solute and the path length of light through the solution.
-
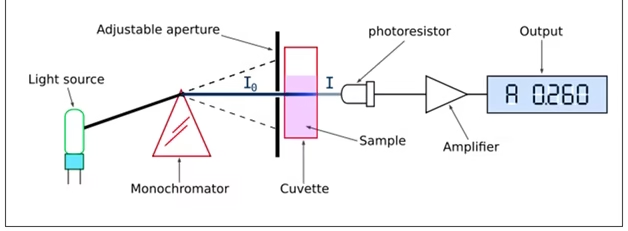
The spectrophotometer uses a light source that passes through a monochromator to select a specific wavelength of light.
-
The selected light passes through the sample, and the detector measures the amount of light transmitted or absorbed.
-
The difference between the incident and transmitted light gives the absorbance value of the sample.
-
It can measure both visible and ultraviolet light depending on the design and wavelength range of the instrument.
-
The results are displayed digitally as absorbance or transmittance, which can be used to calculate the concentration of unknown samples.
-
Spectrophotometers are widely used in chemistry, biochemistry, clinical laboratories, and research for quantitative analysis of colored and colorless compounds.
Working Principle

The operation of a spectrophotometer is based on Beer-Lambert’s Law, which states that the absorbance (A) of a solution is directly proportional to the concentration (C) of the absorbing species in the solution and the path length (l) of the light passing through the solution:
A = ε⋅ C⋅ l
Where:
- A = absorbance,
- ε = molar absorptivity or extinction coefficient
- C = concentration of the solution,
- l = path length (usually the width of the cuvette, typically 1 cm).
Components
Light Source:
The light source provides continuous, stable radiation over the required wavelength range. It is the heart of the instrument since the accuracy of absorbance readings depends on its intensity and stability.
-
For visible region (400–700 nm): a tungsten filament lamp is used because it emits strong, steady light in this range.
-
For ultraviolet region (190–400 nm): a deuterium discharge lamp or hydrogen lamp is preferred, as it gives continuous UV radiation.
-
For infrared region (700–2500 nm): sources like Nernst glower, globars, or mercury lamps may be used.
-
Modern spectrophotometers often have dual lamps, automatically switching between UV and visible ranges to provide seamless scanning.
Entrance Slit:
- The entrance slit is a narrow opening through which the light beam enters the monochromator.
- Its function is to allow a controlled, narrow, and parallel beam of light, preventing stray light from entering.
- A well-adjusted slit improves spectral resolution (ability to distinguish between close wavelengths).
- If the slit is too wide, it allows more light but reduces resolution; if too narrow, it increases resolution but reduces light intensity.
Monochromator:
- The monochromator isolates a single wavelength of light from the polychromatic source.
- It disperses light into its component wavelengths and directs the selected one toward the sample.
- It consists of three main elements:
-
-
Entrance slit – receives light from the source.
-
Dispersing element – either a prism (uses refraction) or a diffraction grating (uses diffraction and interference).
-
Exit slit – selects the desired wavelength from the dispersed spectrum.
The use of a diffraction grating gives high accuracy and reproducibility in modern spectrophotometers.
-
Wavelength Selector / Filter System:
- The wavelength selector controls the exact wavelength reaching the sample by adjusting the prism or grating angle.
- Older instruments used interference filters for fixed wavelength ranges, while modern devices have motor-driven selectors for continuous wavelength scanning.
- The chosen wavelength corresponds to the analyte’s maximum absorbance (λmax), ensuring maximum sensitivity and minimal error.
Sample Holder (Cuvette):
The cuvette is a small rectangular container that holds the liquid sample. It is positioned in the light path so that the beam passes directly through it.
-
-
Glass cuvettes are used for visible light measurements.
-
Quartz or fused silica cuvettes are required for UV region, as glass absorbs UV light.
-
The path length (distance light travels through the sample) is usually 1 cm, but special cuvettes may have shorter or longer paths.
The cleanliness and optical quality of the cuvette are crucial; even fingerprints or scratches can cause absorbance errors.
-
Reference (Blank) Cuvette:
- Alongside the sample cuvette, a reference cuvette containing only the solvent or reagent blank is used.
- The blank sets the baseline (zero absorbance) so that any light absorption is attributed solely to the analyte.
- In double-beam spectrophotometers, light is automatically split between the reference and sample cuvettes for simultaneous correction of any lamp fluctuation.
Detector (Photoelectric System):
- The detector converts transmitted light into an electrical signal proportional to its intensity.
- Types of detectors include:
-
-
Photovoltaic cells: generate current directly by light energy; simple but less sensitive.
-
Phototubes: use a photoemissive surface that emits electrons when struck by light.
-
Photomultiplier tubes: highly sensitive detectors that amplify electron flow, suitable for low-intensity light.
The detector’s sensitivity and linear response are essential for accurate quantitative analysis.
-
Amplifier:
- The electrical signal from the detector is often too weak to be measured directly.
- The amplifier strengthens this signal to a usable level without introducing noise or distortion.
- Modern instruments use solid-state amplifiers with automatic gain control, ensuring stable and precise readings across a wide range of concentrations.
Readout / Display System:
- The amplified signal is processed and displayed as either absorbance (A) or percent transmittance (%T).
- Older models used analog meters or chart recorders, while digital spectrophotometers display readings on LCD screens or computer interfaces.
- In scanning spectrophotometers, the instrument automatically plots absorbance versus wavelength, giving a complete absorption spectrum of the compound.
Power Supply and Control Unit:
- The power supply provides constant, regulated electrical energy to maintain the lamp’s brightness and system stability.
- The control unit houses operational knobs or buttons for wavelength adjustment, calibration, mode selection (absorbance, transmittance, concentration), and automatic zero setting.
Computer Interface and Data Processing Unit (in modern models):
Advanced spectrophotometers are microprocessor-based. They are connected to a computer for:
-
Storing calibration curves and results.
-
Plotting spectra and calculating λmax automatically.
-
Performing advanced analyses such as kinetic studies, multi-component analysis, and baseline corrections.
These systems enhance accuracy, reproducibility, and data management.
Additional Optical Components (in Double-Beam Instruments):
-
Beam Splitter: divides the light beam into two paths—one for the reference and one for the sample.
-
Chopper: alternately allows each beam to reach the detector for comparison.
This configuration compensates for lamp intensity changes, ensuring high stability and precision.
Types of Spectrophotometers
- UV-Vis Spectrophotometer:
- Measures light absorption in the ultraviolet and visible regions (typically 200 nm to 800 nm).
- Widely used in chemistry and biology to determine DNA, proteins, and other molecules concentrations.
- IR Spectrophotometer:
- Measures light absorption in the infrared region (typically 700 nm to 1 mm).
- Used in materials science, environmental monitoring, and chemistry for identifying organic and inorganic compounds.
- Atomic Absorption Spectrophotometer:
- Analyzes the concentration of metal ions in a sample by measuring the amount of light absorbed by free atoms in the gas phase.
- Widely used in environmental testing, food safety, and clinical applications.
Applications
-
Quantitative Analysis:
It is widely used to determine the concentration of unknown solutions by measuring absorbance and comparing it with a standard calibration curve. -
Qualitative Analysis:
By recording the absorption spectrum, it helps identify substances based on their characteristic wavelength of maximum absorption (λmax). -
Biochemical and Clinical Applications:
-
Measurement of blood glucose, urea, creatinine, bilirubin, cholesterol, and enzymes.
-
Used for protein estimation (e.g., Biuret, Lowry, or Bradford methods) and nucleic acid quantification at 260 nm.
-
Helps in enzyme kinetics and determination of enzyme activity by monitoring the rate of color change.
-
-
Pharmaceutical Analysis:
Used to analyze drugs and formulations for purity, concentration, and stability testing.
It helps in dissolution studies, drug release kinetics, and detection of degradation products. -
Environmental Monitoring:
-
Estimation of pollutants like nitrates, phosphates, heavy metals, and chlorophyll content in water samples.
-
Measurement of air and water quality parameters using colorimetric assays.
-
-
Food and Beverage Industry:
Used to test color intensity, vitamin content, and purity of food products.
It measures pigments, antioxidants, and additives to ensure quality control. -
Microbiology and Molecular Biology:
-
Determination of cell density (OD at 600 nm) to monitor bacterial growth.
-
Used in DNA, RNA, and protein quantification.
-
Assessment of enzyme-substrate reactions in biochemical assays.
-
-
Industrial Applications:
Applied in paint, dye, textile, and chemical industries to measure color strength, transparency, and quality of materials. -
Forensic Science:
Helps in the analysis of blood stains, drugs, and toxic substances, based on their absorption spectra. -
Research and Academic Studies:
Extensively used in biochemical, chemical, and pharmacological research for reaction monitoring, compound identification, and studying chemical kinetics. -
Plant and Agricultural Studies:
Used for determining chlorophyll and carotenoid content, nitrate estimation, and plant nutrient analysis. -
Clinical Diagnostic Kits:
Most automated analyzers in pathology labs are spectrophotometer-based instruments that measure light absorbance to report biochemical parameters rapidly.
Advantages
-
High Accuracy and Precision:
It provides accurate and reproducible quantitative results for both colored and colorless solutions. -
Wide Range of Applications:
It can measure substances in the UV, visible, and infrared regions, making it useful in chemistry, biochemistry, medicine, and industry. -
Sensitive and Reliable:
Even small concentration changes can be detected due to the high sensitivity of detectors and stable light sources. -
Rapid and Convenient:
Measurements are quick and require only a few seconds once the instrument is calibrated. -
Small Sample Volume Required:
Only a small amount of solution (usually 1–3 mL) is needed for analysis, which is useful for biological or precious samples. -
Non-Destructive Technique:
The sample remains intact after measurement and can be reused for further testing. -
Quantitative and Qualitative Use:
It not only measures concentration but also helps identify substances through their characteristic absorption spectra. -
Automation and Data Storage:
Modern spectrophotometers are computerized, allowing automatic scanning, data analysis, and spectral plotting. -
Enhanced Stability in Double-Beam Instruments:
Double-beam models automatically correct for fluctuations in light intensity, improving accuracy.
Disadvantages
-
Costly Equipment:
High-quality spectrophotometers, especially UV-visible and double-beam types, are expensive to purchase and maintain. -
Requires Skilled Operation:
Accurate handling, calibration, and wavelength selection require trained personnel to avoid errors. -
Interference Errors:
Presence of impurities, bubbles, or turbidity in the solution can scatter light and cause inaccurate readings. -
Sample Preparation Needed:
Samples must be clear, homogeneous, and sometimes chemically treated before measurement. -
Limited to Transparent or Homogeneous Samples:
Opaque, highly turbid, or solid samples cannot be analyzed directly without modification. -
Instrument Drift and Lamp Aging:
Over time, the light source intensity and detector sensitivity may change, leading to calibration errors. -
Temperature Sensitivity:
Changes in ambient temperature can affect absorbance values, especially for enzyme or biological assays. -
Requires Regular Calibration and Maintenance:
To maintain accuracy, frequent standardization with blanks and known standards is necessary.
Important Considerations
- Calibration: Proper calibration using a blank sample (containing only the solvent) is essential for accurate measurements.
- Wavelength Selection: Choosing the correct wavelength is crucial, as different compounds absorb light at different wavelengths.
- Sample Purity: Impurities in the sample can lead to inaccurate readings.
- Maintenance: Regular cleaning and maintenance of cuvettes and optical components ensure reliable results.