Introduction
- The principle of staining methods in the microbiology lab, like Gram staining, is a fundamental laboratory technique used to classify bacteria into two major groups: Gram-positive and Gram-negative.
- Developed by Hans Christian Gram in 1884, this staining method is crucial for identifying bacterial species and informing treatment options for infections.
Principle of Gram stain

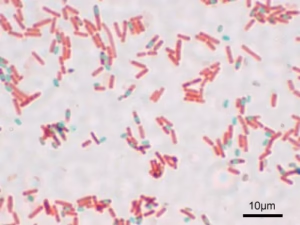
The Gram stain is a differential staining technique that classifies bacteria into two groups based on their cell wall composition: Gram-positive and Gram-negative. The principle is based on the ability of the cell wall to retain the crystal violet stain during a decolourization step.
- Gram-positive bacteria have a thick peptidoglycan layer that retains the crystal violet-iodine complex, appearing purple.
- Gram-negative bacteria have a thinner peptidoglycan layer and an outer membrane, which allows the dye to wash out during decolourization, appearing pink after counterstaining.
Requirements
- Microscope: Light microscope for visualization.
- Glass slides: For preparing bacterial smears.
- Bunsen burner: For sterilization and flame fixation.
- Inoculating loop: For transferring bacteria.
Reagents
- Crystal Violet (Primary Stain): A basic dye that stains all cells purple.
- Concentration: 0.1% solution in distilled water.
- Iodine (Mordant): Forms a complex with crystal violet, enhancing its retention.
- Concentration: 0.1% solution of iodine in distilled water.
- Decolorizer: Usually ethanol or acetone, used to differentiate between Gram-positive and Gram-negative bacteria.
- Safranin (Counterstain): A red dye that stains Gram-negative bacteria after decolourization.
- Concentration: 0.1% solution in distilled water.
Procedure
- Prepare a Bacterial Smear:
- Place a small drop of water on a glass slide.
- Using an inoculating loop, transfer a small amount of the bacterial culture to the drop and spread it.
- Allow the smear to air dry and then heat-fix by passing it through a flame.
- Staining Steps:
- Primary Stain: Flood the slide with crystal violet for 1 minute, then rinse gently with water.
- Mordant: Apply iodine for 1 minute, then rinse with water. This forms the complex with the crystal violet.
- Decolourization: Add ethanol dropwise until no more purple colour runs off (usually a few seconds). Rinse immediately with water.
- Counterstain: Flood the slide with safranin for 30 seconds, then rinse with water.
- Observation:

- Gently blot the slide dry and observe under a microscope using the oil immersion lens.
Results and Interpretation
- Gram-positive Bacteria:
- Stain Purple: Indicate a thick peptidoglycan layer, e.g., Staphylococcus aureus.
- Gram-negative Bacteria:
- Stain Pink: Indicate a thin peptidoglycan layer and an outer membrane, e.g., Escherichia coli.
- Non-staining Organisms:
- Some bacteria, such as Mycobacteria, may not stain well and require acid-fast staining due to their unique cell wall composition.
Applications of Gram Staining
- Bacterial Identification:
- Helps in the initial classification of bacteria as Gram-positive or Gram-negative, aiding in identifying unknown isolates in clinical and environmental samples.
- Diagnosis of Infections:
- Used in clinical microbiology to quickly diagnose bacterial infections, guiding treatment decisions (e.g., using antibiotics).
- Guiding Treatment:
- Identifying Gram-negative vs. Gram-positive bacteria helps clinicians choose appropriate antibiotic therapies, as many antibiotics are more effective against one group.
- Understanding Pathogenicity:
- Gram staining can provide insight into the potential pathogenicity of organisms. For example, certain Gram-negative bacteria are known to be more virulent due to their outer membrane.
- Research Applications:
- Used in microbiological research to study bacterial physiology, genetics, and the effects of environmental changes on bacterial populations.
- Quality Control:
- In industries like food and pharmaceuticals, Gram staining can be part of quality control processes to monitor microbial contamination.
- Environmental Microbiology:
- Used in assessing the microbial composition of environmental samples, such as soil or water, providing insights into ecosystem health.
- Education:
- A fundamental teaching tool in microbiology labs, demonstrating basic staining techniques, cell morphology, and bacterial classification.
- Biofilm Studies:
- It is useful in studying biofilms, as the Gram stain can help visualize and differentiate between microbial communities on surfaces.
Acid-Fast Bacilli Staining
- Acid-fast bacilli (AFB) staining is a crucial technique used primarily to identify mycobacteria, such as Mycobacterium tuberculosis, which are responsible for tuberculosis and other infections.
- Here’s an overview of its principles, requirements, reagents, procedure, results, and applications.
Principle
- AFB staining relies on mycobacteria’s unique cell wall composition, which contains mycolic acids.
- These lipids make the bacteria resistant to decolourization by acid-alcohol after being stained with a specific dye.
- Thus, acid-fast organisms retain the initial dye even after exposure to an acid solution.
Requirements
- Microscope: Light microscope for observation.
- Glass slides: For preparing smears.
- Bunsen burner: For sterilization and flame fixation.
- Inoculating loop: For transferring bacterial samples.
Reagents
- Carbol Fuchsin (Primary Stain):
- A strong red dye that penetrates the cell wall.
- Usually heated to enhance penetration.
- Acid-Alcohol (Decolorizer):
- A mixture of hydrochloric acid and ethanol (typically 3% HCl in 95% ethanol) removes the stain from non-acid-fast organisms.
- Methylene Blue (Counterstain):
- A blue dye is used to stain non-acid-fast organisms, providing contrast.
Procedure
- Prepare a Bacterial Smear:
- Place a small amount of the bacterial culture on a glass slide, spread it, and let it air dry.
- Heat-fix the slide by passing it through a flame.
- Staining Steps:
- Primary Stain: Flood the slide with carbol fuchsin and heat gently for 5 minutes to allow the dye to penetrate. Let it cool, then rinse with water.
- Decolourization: Apply the acid-alcohol dropwise for 1-2 minutes or until no more red colour runs off. Rinse immediately with water.
- Counterstain: Flood the slide with methylene blue for 30 seconds, then rinse with water.
- Observation:

- Blot the slide dry under a microscope using the oil immersion lens.
Results and Interpretation
- Acid-Fast Bacteria:
- Appear red due to retention of the carbol fuchsin stain (e.g., Mycobacterium tuberculosis).
- Non-Acid-Fast Bacteria:
- Appear blue after being counterstained with methylene blue.
Applications
- Diagnosis of Tuberculosis:
- The AFB stain is crucial for diagnosing pulmonary and extrapulmonary tuberculosis by examining sputum samples.
- Identification of Mycobacterial Infections:
- Used to identify other mycobacterial species responsible for infections, such as Mycobacterium leprae (leprosy) and non-tuberculous mycobacteria.
- Research:
- Useful in studying the biology and pathogenicity of mycobacteria in research settings.
- Quality Control:
- Employed in clinical labs to monitor samples for the presence of mycobacteria.