
Introduction
- The embedding process and the choice of embedding media are critical for achieving high-quality histological sections, essential for accurate diagnosis and research.
- In histology, various moulds are used for embedding tissue samples in different embedding media, most notably paraffin wax.
- Moulds help shape the embedded tissue into blocks easily sectioned for microscopic examination.
- Here’s an overview of the different types of moulds used in histology:
Moulds
1. Metal Moulds
- Material: Usually made of stainless steel or aluminium.
- Description: These moulds are durable and can withstand high temperatures, making them suitable for use with molten paraffin.
- Types:
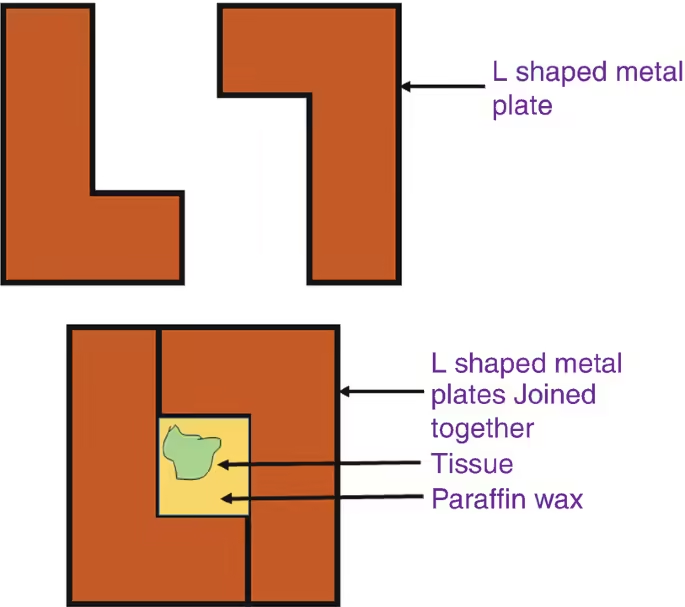
- Flat Moulds: Used for embedding larger tissue or multiple small samples together.
- Cavity Moulds: Designed for specific shapes, such as small biopsies or delicate specimens.
- Advantages:
- Durable and reusable.
- Good heat conductivity, allowing for even cooling of the paraffin.
- Disadvantages:
- It can be heavy and may require careful handling.
2. Plastic Moulds
- Material: Made from various types of plastic (e.g., polystyrene, polypropylene).
- Description: These lightweight moulds can be disposable or reusable depending on the type.
- Types:
- Single-use Moulds: Often used for small biopsies or routine embedding procedures to avoid cross-contamination.
- Reusable Moulds: Designed for multiple uses, often requiring thorough cleaning between uses.
- Advantages:
- Cost-effective, especially when single-use.
- Lightweight and easy to handle.
- Disadvantages:
- It may not be as heat-conductive as metal moulds.
- It can deform under high temperatures.
3. Silicone Moulds
- Material: Made from silicone rubber.
- Description: Flexible moulds that can accommodate complex shapes and contours of tissue samples.
- Types:
- Flexible Moulds: Allow easy removal of embedded samples without damaging the tissue.
- Advantages:
- A non-stick surface helps in the easy removal of paraffin blocks.
- It can be used for a variety of tissue types and shapes.
- Disadvantages:
- It may not withstand as high temperatures as metal moulds.
- Potential for wear and tear over time.
4. Ceramic Moulds
- Material: Made from ceramic materials that can endure high temperatures.
- Description: Less commonly used but suitable for embedding applications that require high heat resistance.
- Advantages:
- Excellent thermal stability.
- It can provide a smooth surface for tissue embedding.
- Disadvantages:
- Fragile and can break easily if dropped.
- Generally heavier and more expensive than plastic moulds.
5. Custom Moulds
- Material: Can be made from various materials based on specific needs (e.g., silicone, metal).
- Description: Tailored moulds designed for research projects or tissue types.
- Advantages:
- It can accommodate unique shapes or larger specimens.
- Designed to fit specific study requirements.
- Disadvantages:
- Typically, more costly to produce.
- It may not be readily available for general use.
Embedding media
Embedding media in histology depends on various factors, including the type of tissue, the desired microscopic analysis, and the specific staining techniques. Each embedding medium has unique advantages and limitations, making selecting the appropriate one for optimal results in histological studies essential.
1. Paraffin Wax
- Composition:
- Paraffin wax is primarily made of saturated hydrocarbons. It can come in various melting points, typically from 56°C to 60°C.
- Preparation:
- Dehydration: After fixation, tissues are dehydrated through ascending ethanol concentrations.

- Clearing: The dehydrated tissue is then cleared using xylene or toluene, which removes the alcohol.
- Infiltration: Tissues are infiltrated with melted paraffin at around 60°C, allowing the wax to penetrate completely.
- Embedding: The infiltrated tissue is placed in a mould and cooled to solidify the wax.
- Dehydration: After fixation, tissues are dehydrated through ascending ethanol concentrations.
- Uses:
- Routine histopathology for diagnosing diseases.
- Staining techniques include Hematoxylin and Eosin (H&E) and special stains (e.g., Masson’s trichrome).
- Considerations:
- Before staining, it may require dewaxing steps (using xylene or similar solvents).
- The heating process can potentially damage heat-sensitive structures or antigens.
2. Resin Media
- Epoxy Resin
- Composition:
- Made from epoxide monomers (like Bisphenol A) and hardeners, typically requiring a polymerization step under heat or ultraviolet light.
- Preparation:
- Dehydration and Clearing: Similar to paraffin, but often requires different solvents (like propylene oxide).
- Infiltration: Tissue is immersed in a mixture of epoxy resin and a hardener.
- Polymerization: This mixture is often placed in an oven for polymerization, resulting in a very hard block.
- Uses:
- Electron microscopy and immunohistochemistry, especially for studying ultrastructural details.
- Ideal for hard tissues like bone or dense connective tissue.
- Considerations:
- Requires specialized equipment for sectioning (e.g., ultramicrotome).
- The resin’s hard consistency can make it difficult to obtain ultra-thin sections (less than 1 micrometre).
b. Acrylic Resin
- Composition:
- Typically involves methacrylate compounds that polymerize in situ, often under UV light.
- Preparation:
- Similar to epoxy resins but may use different polymerization techniques.
- Uses:
- Good for high-resolution imaging in both light and electron microscopy.
- Suitable for immunohistochemical techniques.
- Considerations:
- More expensive than paraffin and epoxy, requiring careful handling during embedding and sectioning.
3. Cryoembedding Media
- Composition:
- OCT compound is a common cryoembedding medium made of polyvinyl alcohol and other polymers that provide a viscous environment.
- Preparation:
- Tissue samples are typically snap-frozen in liquid nitrogen or on dry ice and then embedded in the OCT medium.
- Uses:
- Provides rapid processing for immunofluorescence studies and enzyme histochemistry.
- Allows better antigenicity and enzyme activity preservation than formalin-fixed, paraffin-embedded tissues.
- Considerations:
- Sections may need to be cut quickly to avoid thawing.
- Thicker sections (typically 10-20 micrometers) may not be suitable for some high-resolution imaging techniques.
4. Gelatin and Agar
- Composition:
- Gelatin is derived from collagen, while agar is derived from red algae (agarose).
- Preparation:
- Tissues are immersed in molten gelatin or agar, cooled, and then solidified.
- Uses:
- Useful for embedding delicate tissues that may distort in harder media.
- Commonly used in enzyme histochemistry as it allows for better enzyme activity retention.
- Considerations:
- Limited longevity may not support long-term storage of samples.
- Generally used for specific studies rather than routine histology.
5. Fischer’s Fixative and Paraformaldehyde
- Composition:
- Fischer’s fixative typically comprises formaldehyde in a buffered solution (often phosphate buffer) with Methanol.
- Preparation:
- Tissues are fixed in the solution before dehydrating and embedded in paraffin or resin.
- Uses:
- Common in light and electron microscopy, especially for preserving cellular morphology.
- Considerations:
- Care must be taken with exposure to formaldehyde due to its toxic nature.
Additional Considerations for Embedding Media
- Choice of Media: The choice of embedding medium depends on factors like tissue type, the goal of the study (diagnostic vs. research), and the subsequent staining techniques.
- Storage and Handling: Proper storage conditions (e.g., temperature control) are essential for preserving the embedding media and the embedded tissue.
- Compatibility with Staining: Some media can interfere with certain staining techniques, so understanding the specific requirements of the histochemical methods planned for use is critical.