Introduction
- The Hemagglutination Inhibition assay (HAI) is a serological technique widely used in virology to detect and quantify antibodies against viruses possessing hemagglutinin proteins.
- This method is especially used for evaluating immune responses to influenza, measles, rubella, dengue, and other viruses that naturally cause hemagglutination of red blood cells (RBCs).
- It is a simple, cost-effective, and highly specific method that provides critical insights into viral infections, vaccine efficacy, and population immunity.
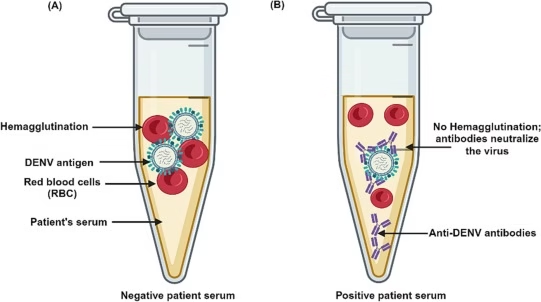
- The principle of this assay is based on the inhibition of hemagglutination by antibodies present in a serum sample.
- When a virus with hemagglutinin proteins is introduced to RBCs, it binds to their surface, leading to RBCs clumping (agglutination).
- However, if a serum sample contains specific antibodies against the virus, these antibodies bind to the viral hemagglutinin, preventing it from interacting with RBCs and inhibiting hemagglutination.
- The highest dilution of the serum that prevents hemagglutination is recorded as the antibody titer.
- This assay is crucial in epidemiology, clinical diagnostics, and vaccine research.
- It helps determine previous exposure to a virus and the effectiveness of vaccination programs by measuring antibody levels in individuals or populations.
Principle
The HAI assay is based on the principle of virus-mediated hemagglutination and its inhibition by virus-specific antibodies.
- Hemagglutination Mechanism: Certain viruses like influenza and rubella have surface glycoproteins called hemagglutinins. These proteins specifically bind to sialic acid-containing receptors on the surface of RBCs, causing the cells to clump together (hemagglutination). This clumping can be visually observed as a diffuse mat of RBCs in a microtiter plate well.
- Inhibition of Hemagglutination: If a serum sample contains antibodies against the viral hemagglutinin, these antibodies will bind to the viral particles, neutralizing them and preventing them from attaching to RBCs. This forms a distinct button-like pellet at the bottom of the well, indicating hemagglutination inhibition.
- Quantification of Antibody Titer: The serum sample is serially diluted, and each dilution is tested for its ability to inhibit hemagglutination. The highest dilution of serum that completely prevents hemagglutination represents the antibody titer. Higher titers indicate stronger immune responses.
Procedure
- Preparation of Reagents
- Viral Antigen: A standardized virus suspension with known hemagglutination activity is prepared. This may be an inactivated or live virus.
- Serum Sample: Patient serum is collected and heat-inactivated at 56°C for 30 minutes to eliminate nonspecific inhibitors that may interfere with the assay.
- RBC Suspension: A 0.5%–1% suspension of RBCs from a species susceptible to the virus (e.g., chicken, guinea pig, or turkey) is prepared in phosphate-buffered saline (PBS).
- Serial Dilution of Serum
- The serum is diluted in a series of twofold dilutions (e.g., 1:10, 1:20, 1:40, etc.) in microtiter plate wells containing buffer.
- Each dilution provides a quantitative measure of the antibody concentration in the sample.
- Addition of Viral Antigen
- A fixed amount of virus is added to each well and incubated at room temperature for about 30–60 minutes.
- During this step, antibodies in the serum (if present) bind to the virus, preventing it from interacting with RBCs.
- Addition of RBC Suspension
- RBCs are added to each well and gently mixed.
- The plate is incubated for another 30 minutes to allow hemagglutination or its inhibition to occur.
- Result Interpretation
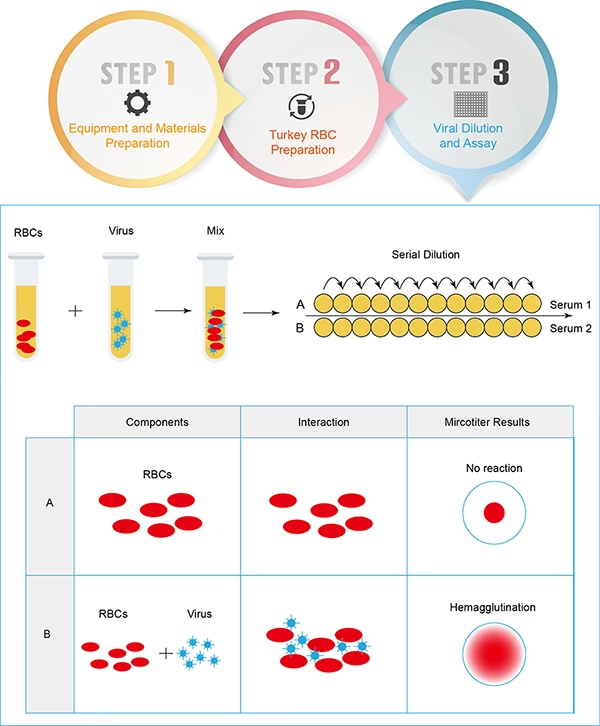
- Positive HAI (Inhibition of Hemagglutination): A distinct button-like pellet of RBCs forms at the bottom of the well, indicating the presence of virus-neutralizing antibodies.
- Negative HAI (No Inhibition of Hemagglutination): A diffuse mat or spread of RBCs indicates hemagglutination due to the absence of specific antibodies.
- The highest dilution of serum that completely inhibits hemagglutination is recorded as the antibody titer.
Advantages
- High Sensitivity and Specificity
- The HAI assay is highly specific for detecting antibodies against hemagglutinating viruses. It allows precise differentiation between infected and non-infected individuals.
- Cost-Effective
- Unlike molecular techniques (e.g., PCR), HAI does not require expensive reagents or specialized equipment, making it suitable for large-scale epidemiological studies.
- Quantitative Analysis
- Provides numerical values (titers) for antibody levels, which can be used for comparative analysis in immunity and vaccine studies.
- Widely Used in Vaccine Studies
- HAI is a standard method for assessing immune response following vaccination, particularly for influenza. It is used to measure the effectiveness of vaccines by comparing antibody titers before and after immunization.
Disadvantages
- Time-Consuming
- The test involves multiple incubation steps and careful sample preparation, making it slower than some modern rapid diagnostic methods.
- Requires Standardization
- The results depend on several factors, including virus concentration, RBC type, incubation time, and serum treatment, which must be carefully standardized for reproducible results.
- Potential for Cross-Reactivity
- Antibodies against one virus may partially inhibit hemagglutination caused by related viruses, leading to false-positive results.
- Not Applicable to All Viruses
- The HAI assay is only useful for viruses that naturally cause hemagglutination. Non-hemagglutinating viruses cannot be tested using this method.
Limitations
- Requires Live or Inactivated Virus
- Handling live viruses poses a biosafety risk, requiring specialized laboratory facilities. Inactivated viruses are sometimes used, but their preparation must be carefully controlled.
- Serum Quality Can Affect Results
- Factors such as hemolysis, lipemia, or the presence of nonspecific inhibitors in serum can interfere with the accuracy of the test.
- Limited Dynamic Range
- The assay may not detect very low antibody concentrations, making it less sensitive than techniques like ELISA or neutralization assays.