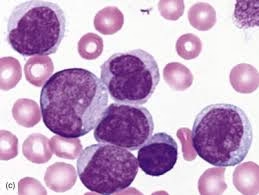
Introduction
-
Di Guglielmo syndrome is a rare and aggressive hematological malignancy involving the erythroid (red blood cell) precursor cells.
-
It is a form of acute myeloid leukemia (AML) and is commonly referred to as erythroleukemia.
-
The disorder is characterized by uncontrolled proliferation of immature erythroblasts in the bone marrow.
-
Normal blood cell production is suppressed, leading to bone marrow failure.
-
It results in severe anemia, often accompanied by leukopenia and thrombocytopenia (pancytopenia).
-
In the FAB classification, it is classified as AML M6.
-
Di Guglielmo syndrome is most commonly seen in elderly patients and often develops secondary to myelodysplastic syndromes or prior chemotherapy.
-
It is considered a clinically aggressive leukemia with poor prognosis.
-
Early diagnosis is essential due to its rapid progression and severe clinical consequences.
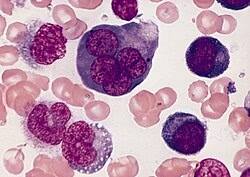
Historical Background
-
Described by Giovanni Di Guglielmo, an Italian pathologist.
-
Initially recognized as a malignant disorder of erythropoiesis.
-
Later incorporated into leukemia classifications due to its aggressive nature.
-
In the FAB classification, it is labeled as AML M6 (Erythroleukemia).
Definition
Di Guglielmo syndrome is an acute leukemia characterized by excessive proliferation of immature erythroid precursor cells in the bone marrow, often associated with myeloblasts, resulting in bone marrow failure.
Classification
FAB Classification
-
AML M6 – Erythroleukemia
WHO Classification (Conceptual Understanding)
-
Considered under acute myeloid leukemia with erythroid predominance
-
Recognized as a biologically aggressive entity
-
Pure erythroid leukemia is separated due to its distinct behavior
Subtypes of Di Guglielmo Syndrome
1. Erythroid/Myeloid Type (M6a)
-
Erythroid precursors > 50% of total marrow cells
-
Myeloblasts ≥ 20% of non-erythroid cells
-
More common subtype
2. Pure Erythroid Leukemia (M6b)
-
Almost complete replacement of marrow by immature erythroblasts
-
Minimal or absent myeloblasts
-
Extremely aggressive with very poor prognosis
Pure erythroid leukemia is considered one of the most malignant leukemias.
Etiology and Risk Factors
The exact cause is unknown, but strong associations include:
-
Secondary leukemia following:
-
Myelodysplastic syndrome (MDS)
-
Aplastic anemia
-
-
Prior chemotherapy or radiotherapy
-
Exposure to:
-
Benzene
-
Ionizing radiation
-
-
Genetic instability
-
Elderly age group (commonly affected)
Most cases arise as transformation from pre-existing marrow disorders.
Pathogenesis
(Initiation → Cellular Transformation → Marrow Infiltration → Disease Evolution)
Initiating Event (Stem Cell Injury)
-
The disease begins with genetic damage to a pluripotent hematopoietic stem cell in the bone marrow.
-
This damage may be primary or secondary (after MDS, chemotherapy, or radiation exposure).
-
The affected stem cell acquires a selective growth advantage.
Genetic and Molecular Alterations
-
Mutations occur in genes regulating:
-
Cell cycle control
-
DNA repair
-
Differentiation pathways
-
-
Common abnormalities include:
-
Complex karyotypes
-
Chromosome 5q and 7q deletions
-
TP53 mutations
-
-
These alterations disrupt normal erythroid maturation.
Lineage Commitment Shift
-
The mutated stem cell shows preferential commitment toward the erythroid lineage.
-
Instead of producing mature red cells, differentiation is arrested at the erythroblast stage.
-
Immature erythroid precursors begin to accumulate abnormally.
Clonal Expansion of Abnormal Erythroblasts
-
The abnormal erythroid precursors undergo uncontrolled clonal proliferation.
-
These cells are:
-
Morphologically abnormal
-
Functionally ineffective
-
-
In some cases, myeloblasts also proliferate, producing the erythroid–myeloid subtype.
Replacement of Normal Marrow Architecture
-
Expanding malignant erythroblasts replace normal hematopoietic tissue.
-
Normal production of RBCs, WBCs, and platelets becomes progressively suppressed.
-
Bone marrow becomes hypercellular but nonfunctional.
Failure of Differentiation and Apoptosis Control
-
Normal apoptotic pathways fail to eliminate abnormal cells.
-
Immature cells survive longer and continue dividing.
-
This leads to ineffective erythropoiesis and marrow exhaustion.
Disease Progression
-
Persistent clonal dominance causes:
-
Pancytopenia
-
Bone marrow failure
-
-
In advanced stages, abnormal erythroid cells spill into the peripheral blood.
-
The disease progresses rapidly with aggressive clinical behavior.
End result: Pancytopenia with severe marrow dysfunction
Pathophysiology
Pathophysiology of Di Guglielmo Syndrome
(Cause → Cellular Events → Systemic Effects → Clinical Outcome)
Marrow-Level Disturbance
-
The bone marrow becomes overloaded with immature erythroid precursor cells.
-
These cells multiply rapidly but fail to mature into functional red blood cells.
-
Although the marrow appears hypercellular, it is functionally ineffective.
Failure of Effective Erythropoiesis
-
Immature erythroblasts dominate the marrow.
-
Normal RBC production is markedly reduced.
-
Result: Severe anemia despite increased cellularity.
Suppression of Other Blood Cell Lines
-
Excess erythroid cells physically crowd out normal precursors.
-
This leads to:
-
↓ White blood cell production → leukopenia
-
↓ Platelet production → thrombocytopenia
-
Imbalance Between Production and Destruction
-
High turnover of abnormal cells increases:
-
LDH
-
Uric acid
-
-
This creates metabolic stress on the body.
Systemic Hypoxia and Compensation
-
Reduced RBCs cause tissue hypoxia.
-
The body responds by:
-
Increasing heart rate
-
Increasing cardiac output
-
-
Clinically manifests as fatigue, breathlessness, and palpitations.
Immune and Hemostatic Failure
-
Leukopenia reduces immune defense → recurrent infections.
-
Thrombocytopenia impairs clot formation → bleeding and petechiae.
Progressive Bone Marrow Failure
-
Continuous ineffective hematopoiesis leads to:
-
Pancytopenia
-
Dependence on transfusions
-
-
In advanced disease, abnormal cells enter circulation.
End-Stage Physiological Consequence
-
Multi-lineage marrow failure
-
Systemic metabolic burden
-
Rapid clinical deterioration if untreated
Clinical Features
Symptoms Due to Anemia
-
Fatigue and weakness
-
Pallor
-
Dyspnea on exertion
-
Palpitations
Symptoms Due to Leukopenia
-
Recurrent infections
-
Fever
-
Poor wound healing
Symptoms Due to Thrombocytopenia
-
Petechiae
-
Easy bruising
-
Epistaxis
-
Gum bleeding
General/Systemic Features
-
Weight loss
-
Fever of unknown origin
-
Hepatosplenomegaly (variable)
Clinical presentation often mimics severe anemia or MDS, delaying diagnosis.
Laboratory Findings
Peripheral Blood Findings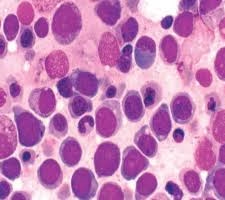
-
Severe anemia
-
Usually normocytic or macrocytic
-
-
Reduced hemoglobin level
-
Leukopenia or leukocytosis
-
Often leukopenia due to marrow suppression
-
-
Thrombocytopenia
-
Presence of nucleated red blood cells (NRBCs)
-
Circulating immature erythroblasts
-
Marked anisopoikilocytosis
-
Reticulocyte count is usually low (ineffective erythropoiesis)
Key clue: Severe anemia with circulating erythroblasts
Bone Marrow Examination 
Bone Marrow Aspiration / Biopsy
-
Hypercellular marrow
-
Erythroid precursors > 50% of total nucleated cells
-
Marked erythroid hyperplasia
-
Megaloblastoid changes in erythroblasts
-
Multinucleated and dysplastic erythroid cells
-
Suppression of normal myeloid and megakaryocytic lines
-
Increased myeloblasts in erythroid–myeloid type (M6a)
Diagnostic hallmark of the disease
Cytochemical Staining
-
PAS (Periodic Acid–Schiff):
-
Strong block positivity in erythroblasts
-
-
Myeloperoxidase (MPO):
-
Positive in myeloblasts (if present)
-
Negative in pure erythroid leukemia
-
PAS positivity helps differentiate from other leukemias
Immunophenotyping (Flow Cytometry)
Erythroid Markers (Positive)
-
CD71 (transferrin receptor)
-
Glycophorin A
-
CD36
Myeloid Markers (Variable)
-
CD13
-
CD33
-
CD117
Confirms erythroid lineage involvement
Cytogenetic & Molecular Findings
-
Complex karyotype
-
Deletions of chromosome 5q and 7q
-
TP53 mutations
-
Monosomal karyotype (poor prognosis)
Associated with aggressive disease and poor outcome
Biochemical Findings
-
↑ Lactate dehydrogenase (LDH)
-
↑ Uric acid (high cell turnover)
-
Possible electrolyte imbalance (tumor lysis risk)
Coagulation Profile
-
Prolonged bleeding time (due to thrombocytopenia)
-
Increased risk of hemorrhage
These abnormalities are associated with poor prognosis.
Differential Diagnosis
| Condition | Key Difference |
|---|---|
| Megaloblastic anemia | No malignant blasts |
| Myelodysplastic syndrome | Less aggressive, lower blast count |
| Other AML subtypes | Predominant myeloid lineage |
| Aplastic anemia | Hypocellular marrow |
Treatment
Management follows AML protocols:
Definitive Treatment
-
Intensive combination chemotherapy
-
Hematopoietic stem cell transplantation (selected patients)
Supportive Care
-
RBC and platelet transfusions
-
Broad-spectrum antibiotics
-
Management of infections
-
Nutritional support
Pure erythroid leukemia responds poorly to therapy.
MCQs
1. Di Guglielmo syndrome is also known as:
A. Myeloblastic leukemia
B. Lymphoblastic leukemia
C. Erythroleukemia
D. Megaloblastic anemia
2. Di Guglielmo syndrome belongs to which leukemia group?
A. Acute lymphoblastic leukemia
B. Chronic myeloid leukemia
C. Acute myeloid leukemia
D. Chronic lymphocytic leukemia
3. In FAB classification, Di Guglielmo syndrome is:
A. M2
B. M4
C. M6
D. M7
4. The primary cell lineage involved in Di Guglielmo syndrome is:
A. Lymphoid
B. Myeloid
C. Erythroid
D. Megakaryocytic
5. Di Guglielmo syndrome was first described by:
A. Virchow
B. Ehrlich
C. Di Guglielmo
D. Hooke
6. The most common age group affected is:
A. Children
B. Adolescents
C. Young adults
D. Elderly
7. Di Guglielmo syndrome commonly develops secondary to:
A. Iron deficiency anemia
B. Myelodysplastic syndrome
C. Thalassemia
D. Hemophilia
8. The hallmark of Di Guglielmo syndrome is:
A. Lymph node enlargement
B. Predominant erythroid proliferation
C. Plasma cell excess
D. Megakaryocyte hyperplasia
9. Which bone marrow finding is diagnostic?
A. Hypocellular marrow
B. Normal cellularity
C. Hypercellular marrow with erythroid predominance
D. Fatty marrow
10. Percentage of erythroid precursors required for diagnosis is:
A. >10%
B. >30%
C. >50%
D. >80%
11. The pure erythroid leukemia subtype is:
A. M6a
B. M6b
C. M5
D. M7
12. In erythroid–myeloid type, myeloblasts are:
A. Absent
B. <5%
C. ≥20% of non-erythroid cells
D. >50%
13. Peripheral blood commonly shows:
A. Polycythemia
B. Pancytopenia
C. Thrombocytosis
D. Leukocytosis only
14. The most prominent clinical feature is:
A. Jaundice
B. Severe anemia
C. Hypertension
D. Edema
15. PAS stain in erythroblasts shows:
A. Negative reaction
B. Diffuse positivity
C. Block positivity
D. Ring positivity
16. PAS positivity helps identify:
A. Lymphoid lineage
B. Myeloid lineage
C. Erythroid lineage
D. Megakaryocytic lineage
17. Myeloperoxidase (MPO) is positive in:
A. Erythroblasts
B. Myeloblasts
C. Lymphoblasts
D. Plasma cells
18. Common erythroid marker in immunophenotyping is:
A. CD3
B. CD20
C. CD71
D. CD41
19. Glycophorin A is a marker of:
A. Myeloid cells
B. Lymphoid cells
C. Erythroid cells
D. Platelets
20. Cytogenetic abnormality commonly associated is:
A. t(9;22)
B. del(5q)
C. t(15;17)
D. t(8;21)
21. TP53 mutation indicates:
A. Good prognosis
B. Viral infection
C. Poor prognosis
D. Drug resistance only
22. Di Guglielmo syndrome results in:
A. Leukocytosis only
B. Isolated anemia
C. Bone marrow failure
D. Splenic rupture
23. Elevated LDH indicates:
A. Liver damage
B. High cell turnover
C. Renal failure
D. Iron deficiency
24. Uric acid levels are increased due to:
A. Reduced excretion
B. Cell lysis
C. Dehydration
D. Diet
25. The reticulocyte count is usually:
A. Increased
B. Normal
C. Decreased
D. Variable
26. Which of the following is a differential diagnosis?
A. Megaloblastic anemia
B. Sickle cell anemia
C. Iron overload
D. Polycythemia vera
27. Megaloblastoid changes are seen in:
A. WBCs
B. Platelets
C. Erythroblasts
D. Lymphocytes
28. Bone marrow cellularity in Di Guglielmo syndrome is:
A. Hypocellular
B. Normocellular
C. Hypercellular
D. Fatty
29. Which cell type is suppressed?
A. Erythrocytes
B. All hematopoietic lineages
C. Only lymphocytes
D. Only granulocytes
30. Thrombocytopenia causes:
A. Hypertension
B. Infections
C. Bleeding tendency
D. Polycythemia
31. Leukopenia leads to:
A. Anemia
B. Fever and infections
C. Bleeding
D. Thrombosis
32. The disease progression is:
A. Slow
B. Benign
C. Aggressive
D. Self-limiting
33. Treatment follows protocols of:
A. ALL
B. CML
C. AML
D. Aplastic anemia
34. Definitive treatment option in selected patients:
A. Splenectomy
B. Bone marrow transplantation
C. Iron therapy
D. Steroids alone
35. Supportive therapy includes:
A. Chemotherapy only
B. Blood transfusions
C. Surgery
D. Radiation alone
36. Prognosis of pure erythroid leukemia is:
A. Excellent
B. Good
C. Fair
D. Poor
37. Disease often evolves from:
A. Thalassemia
B. Iron deficiency anemia
C. Myelodysplastic syndrome
D. Sickle cell disease
38. Peripheral blood shows nucleated RBCs due to:
A. Increased erythropoiesis
B. Marrow stress
C. Marrow infiltration
D. Hemolysis
39. Which enzyme helps differentiate erythroid cells?
A. ALP
B. PAS
C. AST
D. ALT
40. Bone marrow failure leads to:
A. Polycythemia
B. Pancytopenia
C. Leukemoid reaction
D. Thrombocytosis
41. The most aggressive subtype is:
A. M6a
B. M6b
C. M3
D. M5
42. Common clinical triad includes anemia, infection, and:
A. Jaundice
B. Bleeding
C. Hypertension
D. Edema
43. Which lineage shows dysplasia?
A. Only lymphoid
B. Only myeloid
C. Predominantly erythroid
D. Megakaryocytic
44. The marrow appears active but ineffective due to:
A. Hypoplasia
B. Ineffective erythropoiesis
C. Iron deficiency
D. Hemorrhage
45. Di Guglielmo syndrome is best described as:
A. Chronic disorder
B. Benign marrow condition
C. Acute malignant disorder
D. Autoimmune disease
46. A key biochemical marker of tumor burden is:
A. Creatinine
B. LDH
C. Calcium
D. Sodium
47. The condition is often misdiagnosed as:
A. Iron deficiency anemia
B. Megaloblastic anemia
C. Polycythemia
D. Hemolytic anemia
48. The disease affects hematopoiesis by:
A. Enhancing maturation
B. Arresting differentiation
C. Increasing RBC lifespan
D. Improving oxygenation
49. Which organ is primarily affected?
A. Spleen
B. Liver
C. Bone marrow
D. Lymph node
50. The overall survival in Di Guglielmo syndrome is:
A. Long-term
B. Excellent
C. Short
D. Normal
Answer Key
-
C
-
C
-
C
-
C
-
C
-
D
-
B
-
B
-
C
-
C
-
B
-
C
-
B
-
B
-
C
-
C
-
B
-
C
-
C
-
B
-
C
-
C
-
B
-
B
-
C
-
A
-
C
-
C
-
B
-
C
-
B
-
C
-
C
-
B
-
B
-
D
-
C
-
C
-
B
-
B
-
B
-
B
-
C
-
B
-
C
-
B
-
B
-
B
-
C
-
C