Introduction
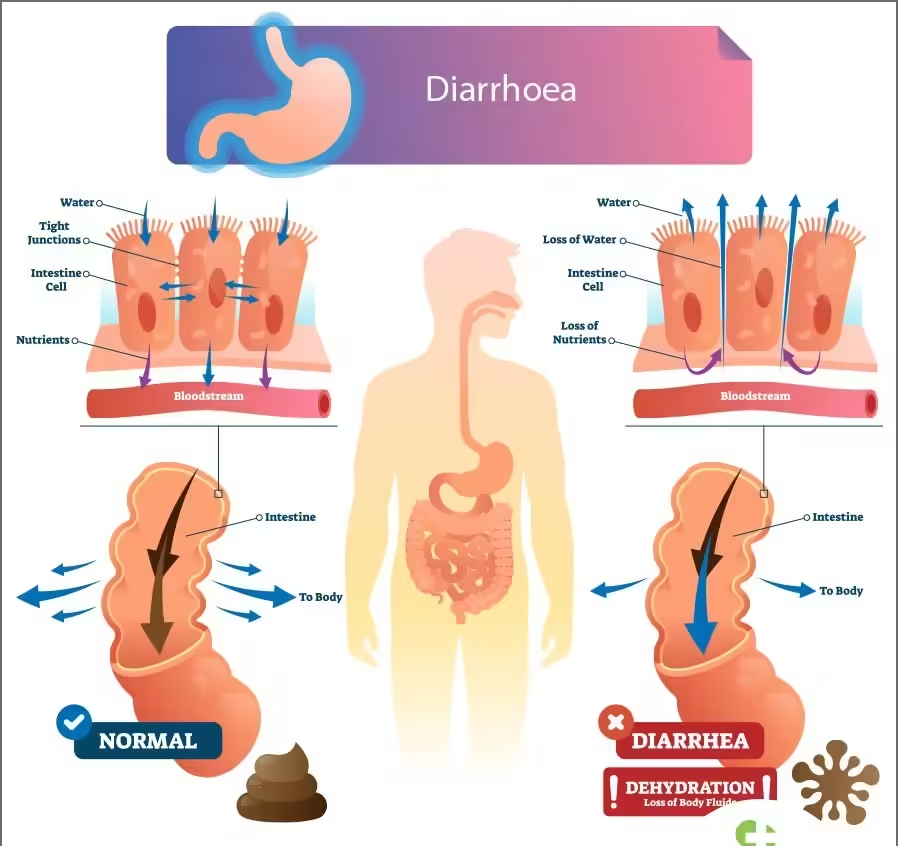
- Acute diarrheal disease is a significant global health issue that can result from various infectious agents, including bacteria, viruses, and parasites.
- It is characterized by the sudden onset of diarrhea, which can lead to dehydration and, in severe cases, death, particularly among vulnerable populations such as children and the elderly.
- Timely and accurate laboratory diagnosis is essential for determining the cause of the diarrhea, guiding appropriate treatment, and implementing effective public health measures.
Clinical Presentation
The clinical features of acute diarrheal disease can vary based on the underlying pathogen. Common symptoms include:
- Diarrhea is defined as the passage of three or more loose or liquid stools daily. The stool may be watery, mucoid, or bloody.
- Abdominal Pain: Cramping and discomfort in the abdominal area.
- Nausea and Vomiting: Often present, especially in viral infections.
- Fever May occur, especially in bacterial infections.
- Dehydration: Results from fluid loss, leading to symptoms such as dry mouth, decreased urine output, and lethargy.
Sample Collection
- Specimen Types
- Stool Samples: The primary specimen for diagnosing acute diarrhea.
- Blood Samples: These may be collected in severe dehydration or systemic illness cases.
- Urine Samples: Occasionally collected to assess dehydration status or to evaluate for concurrent infections.
- Collection Technique
- Stool Collection:
- Collect stool in a clean, sterile container, ideally using a scoop provided by the laboratory.
- Collect the stool sample as soon as diarrhea begins and before starting any antibiotic treatment, as this can affect the culture results.
- For specific tests (e.g., parasitology), collect samples from different days if the diarrhea persists.
- Transport and Handling
- Stool Samples: Should be processed within 24 hours. If transport is delayed, refrigerate samples. Transport media, such as Cary-Blair, can maintain bacterial pathogens’ viability during transport.
Laboratory Techniques for Diagnosis
Stool Culture
A. Selective Media
- Different selective media are used depending on the suspected pathogens:
- MacConkey Agar: Used to isolate Gram-negative bacteria, particularly lactose fermenters and non-fermenters.
- XLD (Xylose Lysine Deoxycholate) Agar: Useful for isolating Salmonella and Shigella species.
- CLED (Cystine Lactose Electrolyte Deficient) Agar: Useful for isolating urinary pathogens.
- Hektoen Enteric Agar: Used for Salmonella and Shigella isolation, differentiating them from other enteric pathogens.
B. Incubation Conditions
- The cultures are incubated at 35-37°C for 24-48 hours. The specific requirements may vary depending on the isolated organism (e.g., anaerobic conditions for Clostridium).
C. Identification of Colonies
- After incubation, colonies resembling suspected pathogens undergo further biochemical testing:
- Indole Production: Differentiates E. coli from other coliforms.
- Urease Test: Used to identify Proteus species.
- Lactose Fermentation: Assessing lactose fermentation can help identify coliforms versus non-lactose fermenters.
Molecular Methods
A. Polymerase Chain Reaction (PCR)
PCR techniques can detect specific pathogens in stool samples rapidly. This method is highly sensitive and specific and is particularly useful for:
-
- Detecting pathogens that are difficult to culture.
- Rapid diagnosis in outbreaks.
B. Multiplex PCR
- This advanced technique allows for the simultaneous detection of multiple pathogens in a single reaction, providing a comprehensive profile of infectious agents responsible for diarrhea.
Antigen Detection Tests
- Enzyme-Linked Immunosorbent Assay (ELISA): Used for the rapid detection of specific antigens from pathogens, such as:
- Rotavirus: A common viral cause of acute gastroenteritis in children.
- C. difficile: Toxin detection for cases associated with antibiotic use.
Serological Tests
- Although less common for acute diarrhea, serological tests can be used in specific contexts, such as identifying antibodies to pathogens like Vibrio cholerae or certain viral infections.
Microscopic Examination
- Direct Microscopy: Fresh stool samples can be examined under a microscope to identify parasites, eggs, or cysts, particularly useful in suspected parasitic infections (e.g., Giardia, Entamoeba histolytica).
- Stool Concentration Techniques: Concentration methods, such as the formalin-ether or zinc sulfate flotation techniques, may be used to enhance the detection of parasites in stool samples.
Interpretation of Results
- Culture Results
- Positive Culture: Isolation of a pathogen from stool confirms the diagnosis. For example:
- Finding Salmonella or Shigella indicates a bacterial infection.
- Vibrio cholerae isolation confirms cholera.
- Negative Culture: A negative result does not rule out infection, particularly if the sample was collected after antibiotic treatment or if the pathogen is non-culturable.
- PCR Results
- Positive PCR Indicates the presence of pathogen-specific DNA, confirming the diagnosis, especially in cases with negative culture results.
- Negative PCR: May not exclude infection, particularly if testing is performed shortly after symptom onset.
- Antigen Detection Tests
- Positive Antigen Test: Confirms the presence of specific pathogens like rotavirus or C. difficile and helps guide treatment decisions.
- Negative Antigen Test: A negative result may not definitively rule out infection, especially in low pathogen loads.
- Microscopy Results
- Identifying parasites, cysts, or eggs in stool samples supports a diagnosis of parasitic infection, providing valuable information for treatment.
Clinical Considerations
Diagnosis and Treatment
- Early and accurate diagnosis is crucial to managing acute diarrheal diseases effectively.
- Rehydration Therapy: Oral rehydration solutions (ORS) are essential for managing dehydration resulting from diarrhea. In severe cases, intravenous fluids may be necessary.
- Antibiotic Therapy: Antibiotics may be indicated for specific bacterial infections (e.g., Shigella, Campylobacter, cholera) but should be used judiciously to avoid unnecessary resistance.
Follow-Up and Monitoring
- Continuous monitoring for dehydration and treatment response signs is important, especially in high-risk populations.
- In cases of persistent diarrhea, further evaluation may be required to rule out other causes or complications.
Public Health Implications
- Acute diarrheal diseases can lead to outbreaks, particularly in poor sanitation and hygiene settings. Public health measures should include:
- Surveillance and reporting of cases.
- Community education on hygiene and sanitation practices.
- Vaccination where applicable (e.g., for rotavirus and cholera).