Amino Acids
- Amino acids are the structural units (monomers) of proteins.
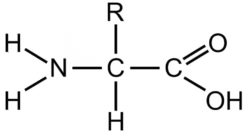
- An amino acid comprises two functional groups—amino (–NH2), and carboxyl (–COOH). They also contain a hydrogen atom and a side chain (R) linked to the carbon atom.
- Amino acids differ from each other in their side chains.
General Structure
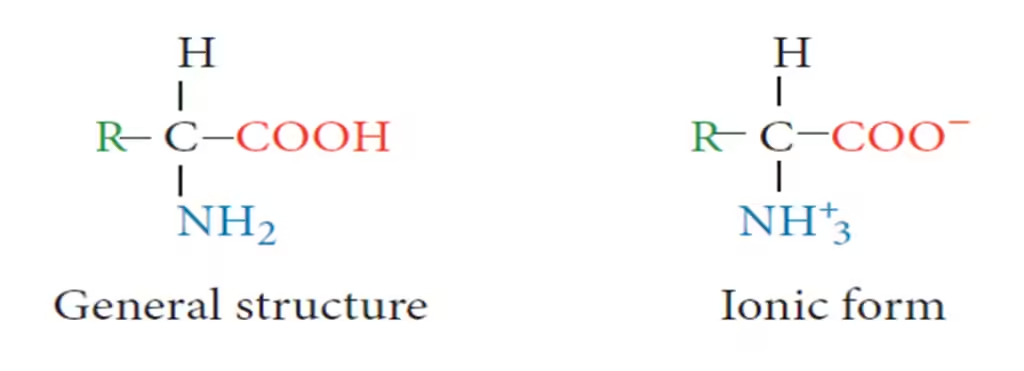
Every amino acid has a basic structure consisting of:
- Central carbon atom: This is often referred to as the alpha-carbon.
- Amino group: A nitrogen-containing group (-NH2). (Basic group)
- Carboxyl group: A carboxylic acid group (-COOH). (Acidic group)
- Hydrogen atom: Attached to the alpha-carbon.
- Sidechain (R group): Each amino acid’s unique part determines its properties and function.
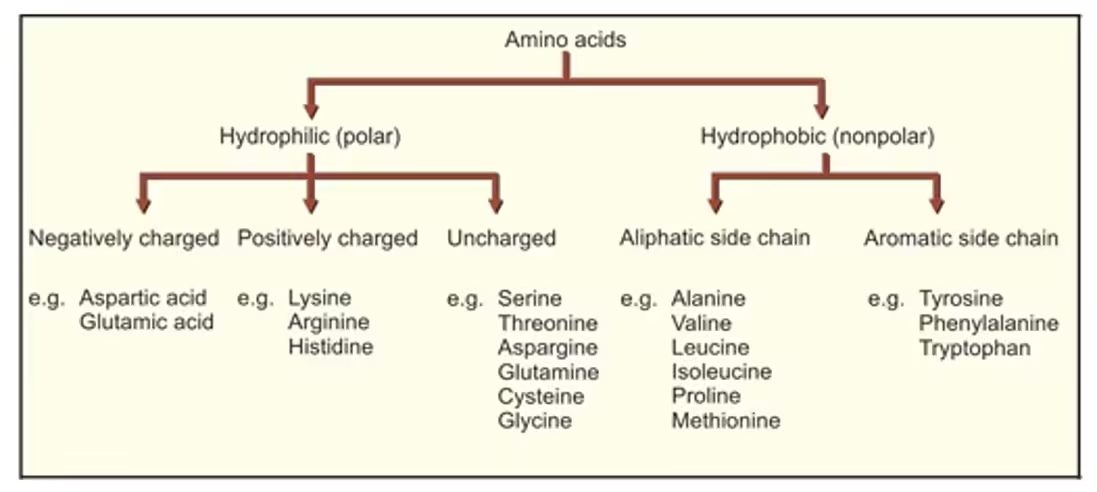
Classification of amino acids
- Based on the variable side chain
- Based on the nutritional requirements of amino acids
- Based on the metabolic products of amino acids
- Based on the nature or polarity of the side chain.
1. Based on the variable side chain
Amino acids with aliphatic side chains (GAVLI)
- Glycine
- Alanine
- Valine
- Leucine
- Isoleucine
Amino acids containing hydroxyl (–OH) groups (ST)
- Serine
- Threonine
Sulphur-containing amino acids (CM)
- Cysteine
- Methionine
Acidic amino acids and their amides (GAGA)
- Aspartic acid
- Asparagine
- Glutamic acid
- Glutamine
Basic amino acids (HAL)
- Lysine
- Arginine
- Histidine
Aromatic amino acids (PTT)
- Phenylalanine
- Tyrosine
- Tryptophan
Imino acid
- Proline
2. Based on nutritional requirements
- Essential – Cannot be synthesised in the body, supplied from diet. Examples are phenylalanine, Valine, Tryptophan, Threonine, Isoleucine, Methionine, Histidine, Arginine, Lysine, and (PVT TIM HALL)
- Semi-essential – Growing children require them in the food, but not essential for adults. Example –
- Nonessential – This can be synthesized in the body, hence not required in the diet. Example – All the other 10 amino acids.
3. Based on metabolic fate
- Ketogenic – Amino Acids that are converted into ketone bodies.
Example – Leucine, Lysine.
- Glucogenic – Amino Acids that enter into glucose.
Example – All the other 14 amino acids
- Both glucogenic and ketogenic – Both are converted into glucose and ketone bodies. Examples are phenylalanine, Isoleucine, Tyrosine, and Tryptophan.
4. Based on the polarity
Biologically important compounds formed by amino acids
| SN. | Amino acid | Biologically important compound |
| 1. | Tyrosine | Hormones, e.g., adrenaline and thyroxine. Skin pigment, e.g., melanin |
| 2. | Glycine, arginine and methionine | Creatine |
| 3. | Glycine and cysteine | Bile salts |
| 4. | Glycine | Heme |
| 5. | Aspartic acid and glutamic acid | Pyrimidine bases |
| 6. | Glycine, aspartic acid and glutamine | Purine bases |
| 7. | β-alanine | Coenzyme-A |
| 8. | Tryptophan | Vitamin, e.g., niacin |
Important of amino acids
- Protein Synthesis
- Enzyme Function
- Hormone Synthesis
- Neurotransmitters
- Energy Source
- Immune Function
- Biosynthesis of Other Molecules
Isoelectric pH
The isoelectric point (pI) of a molecule, particularly an amino acid or protein, is the pH at which the molecule carries no net electrical charge.
Each amino acid contains at least two ionizable groups:
- Amino group (-NH₃⁺)
- Carboxyl group (-COO⁻)
- The amino acid is protonated at low pH (acidic) and has a positive charge.
- The amino acid is deprotonated at high pH (basic) and has a negative charge.
- At the pI, the amino acid has no net charge, which makes it electrically neutral.
Importance of the Isoelectric Point
- Protein Purification
- Solubility
- Biological Function
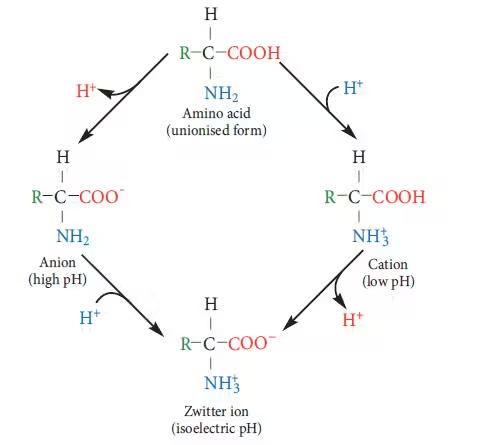
Various ionic forms of amino acids at different pH levels
Zwitterion
A zwitterion is a molecule with both a positive and a negative charge but is neutral overall.
Example: Amino Acids
In water, amino acids can become zwitterions because:
- The amino group (-NH₂) can accept a proton and become positively charged (NH₃⁺).
- The carboxyl group (-COOH) can lose a proton and become negatively charged (-COO⁻).
At a certain pH (near neutral), these charges balance out, resulting in the amino acid becoming a zwitterion—meaning it has both positive and negative charges but no overall charge.
Importance of Zwitterions
- Buffering Capacity
- Solubility
- Protein Structure
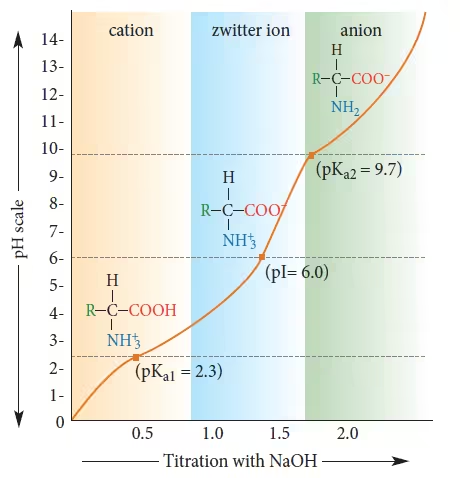
Sorenson’s titration curves of valine
Peptide bond
- A peptide bond is a covalent bond between two amino acids during protein synthesis.
- It is a key linkage in the structure of proteins, holding together long chains of amino acids called polypeptides.
- A condensation reaction forms a peptide bond, where one amino acid’s carboxyl group (-COOH) reacts with another amino acid’s amino group (-NH₂).
Breaking of a Peptide Bond
- Hydrolysis is the reverse process of forming a peptide bond, where water is added to break the bond, releasing individual amino acids.
- This occurs during digestion when proteins are broken down into amino acids by enzymes like proteases.
Importance of Peptide Bonds
- Protein chemistry Structure: Peptide bonds link amino acids in specific sequences to form polypeptides, which fold into functional proteins.
- Enzymatic Function: As seen in digestive enzymes or protein synthesis, many enzymes work by cleaving or forming peptide bonds.
- Biological Signalling: Peptides (short chains of amino acids) can act as hormones or signalling molecules, such as insulin.