Introduction
- C-reactive protein (CRP) is a sensitive biomarker of inflammation, synthesized by the liver in response to cytokines, especially interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin-1 (IL-1).
- CRP plays a role in the immune response by binding to phosphocholine on damaged or dead cells and some microorganisms, promoting their elimination through phagocytosis and complement activation.
- Acute-Phase Response: CRP is part of the acute-phase response, rising quickly (6–8 hours post-stimulus) and peaking at 48 hours. Its levels decrease rapidly once inflammation subsides.
- Clinical Utility: CRP measurement helps identify acute infections, chronic inflammatory conditions, and tissue damage.
Principle of the Test
The CRP test relies on antigen-antibody interaction, where CRP in the patient’s serum reacts with antibodies specific to CRP, leading to agglutination. The intensity of agglutination reflects the CRP level.
Key Assay Techniques:
- Slide Agglutination (Qualitative or Semi-Quantitative):
- Detects the presence or rough estimation of CRP levels.
- Turbidimetric/Immunoturbidimetric:
- Measures the turbidity caused by agglutination, proportional to CRP concentration.
- Nephelometric:
- Measures light scattering due to immune complexes, providing precise CRP levels.
- Enzyme-Linked Immunosorbent Assay (ELISA):
- A highly sensitive method used for specific and quantitative CRP detection.
- High-Sensitivity CRP (hs-CRP):
- Specialized assay for detecting low levels of CRP to assess cardiovascular risk.
Requirements
Sample Collection
- Type: Serum (preferred) or plasma (EDTA/heparinized).
- Volume: 2–3 mL of venous blood.
- Handling:
- Separate serum promptly by centrifugation to avoid hemolysis.
- If testing is delayed, store serum at 2–8°C (up to 72 hours). For longer storage, freeze at −20°C.
- Avoid multiple freeze-thaw cycles, as this can degrade CRP.
Reagents and Materials
- CRP Latex Reagent:
- Polystyrene latex particles coated with anti-CRP monoclonal or polyclonal antibodies.
- Diluent:
- Normal saline or phosphate-buffered saline (PBS).
- Positive Control:
- Serum with a known CRP concentration (used to validate the test).
- Negative Control:
- CRP-free serum or saline (ensures test specificity).
- RBC Suspensions (for older hemagglutination-based methods):
- Used in outdated methods but less common in modern assays.
Equipment
- Glass Slides (for slide tests).
- Rotator (for mixing during slide agglutination).
- Cuvettes (for turbidimetric or nephelometric assays).
- Spectrophotometer/Nephelometer (for quantitative analysis).
- Pipettes and calibrated tips.
Procedure
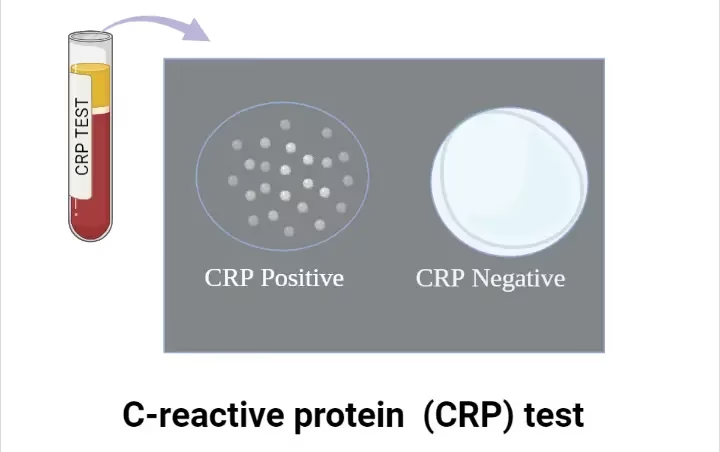
Qualitative Test (Slide Agglutination)
- Place a drop (50 µL) of undiluted serum on a clean glass slide.
- Add an equal drop (50 µL) of CRP latex reagent.
- Mix gently using a disposable stick or slide rotator.
- Observe for visible agglutination within 2–5 minutes.
Interpretation:
- Positive: Visible clumping (CRP present above the detectable limit, often >6 mg/L).
- Negative: No visible clumping (CRP absent or below detectable limit).
Semi-Quantitative Test
- Prepare serial dilutions of the serum (e.g., 1:2, 1:4, 1:8, etc.) in saline or PBS.
- Add 50 µL of each dilution to separate wells or slide areas.
- Add 50 µL of CRP latex reagent to each dilution.
- Mix and observe for agglutination.
- The highest dilution showing agglutination indicates the approximate CRP titer.
Quantitative Test (Immunoturbidimetric or Nephelometric)
- Prepare a calibration curve using CRP standards of known concentrations.
- Mix serum with anti-CRP antibody reagent in a cuvette.
- Incubate the mixture at 37°C for the specified time.
- Measure turbidity (optical density) or light scatter using a spectrophotometer or nephelometer.
- Calculate CRP concentration from the calibration curve.
Results and Interpretation
Normal CRP Levels
- Standard CRP Test: < 10 mg/L indicates no significant inflammation.
- hs-CRP (for Cardiovascular Risk):
- < 1 mg/L: Low risk.
- 1–3 mg/L: Moderate risk.
- > 3 mg/L: High risk.
Elevated CRP Levels
- Mild Elevation (10–40 mg/L):
- Mild infections (e.g., viral infections).
- Pregnancy or obesity-related inflammation.
- Moderate Elevation (40–200 mg/L):
- Active bacterial infections and autoimmune diseases (e.g., rheumatoid arthritis).
- High Elevation (>200 mg/L):
- Severe bacterial infections (sepsis, pneumonia) and significant tissue damage (trauma, burns).
Clinical Significance
- Infections
-
- Elevated CRP levels help distinguish bacterial infections (high CRP) from viral infections (lower CRP).
- Autoimmune Diseases
-
- CRP is elevated in diseases like rheumatoid arthritis, lupus, and vasculitis, serving as a marker of disease activity and treatment response.
- Cardiovascular Diseases
-
- hs-CRP predicts cardiovascular risk by detecting low-level chronic inflammation involved in atherosclerosis.
- Monitoring
-
- CRP levels guide treatment decisions for infections, autoimmune diseases, and post-surgical recovery.
Limitations
- Non-Specific:
- Elevated CRP does not pinpoint the source of inflammation. Clinical correlation is essential.
- False Positives:
- Elevated CRP in pregnancy, obesity, smoking, or hormone replacement therapy.
- Transient Rise:
- CRP levels peak rapidly and normalize quickly; the timing of sample collection is critical.
- Low Sensitivity for Chronic Conditions:
- Conditions with low-grade inflammation (e.g., diabetes, metabolic syndrome) require hs-CRP for detection.
Precautions
- Sample Quality: Avoid hemolyzed or lipemic samples, which may interfere with results.
- Reagent Handling: Store reagents as specified to maintain stability and accuracy.
- Interpretation: Always correlate results with clinical history, physical examination, and other laboratory findings.