Cervical Cytology
- Cervical cytology is a diagnostic and screening method to evaluate cellular changes in the cervix.
- It is primarily performed to detect premalignant lesions and malignancies, allowing early intervention to prevent cervical cancer.
- The commonly performed Pap smear test or liquid-based cytology (LBC) is a worldwide cornerstone of cervical cancer prevention programs.
Introduction
- The cervix is the lower part of the uterus, exposed to multiple factors such as hormonal changes, infections, and carcinogenic agents like human papillomavirus (HPV).
- Cervical cytology evaluates exfoliated cells from the squamous and columnar epithelium to detect:
- Normal cells
- Atypical cells (premalignant lesions)
- Malignant cells (invasive cervical cancer)
- Importance:
Early detection of precancerous lesions through cervical cytology significantly reduces the incidence and mortality of cervical cancer. - Common Indications for Cervical Cytology:
- Routine cervical cancer screening (ages 21–65).
- Evaluation of abnormal vaginal bleeding.
- Follow-up after treatment for cervical dysplasia.
- Monitoring HPV infection in high-risk groups.
Sample Collection Procedure
- Patient Preparation
-
- Ensure the patient is informed about the procedure.
- Advise avoiding the following 24–48 hours before the test:
- Sexual intercourse
- Douching
- Vaginal medications or contraceptive creams
- The test should not be performed during menstruation.
Required Materials
- Speculum (preferably warmed and lubricated with water or saline).
- Cervical sampling devices:
- Ayre’s spatula
- Endocervical brush or broom-like device (e.g., Cervix Brush)
- Glass slide for conventional cytology or liquid-based cytology vial.
- Fixative (spray or liquid-based preservative).
Procedure
- Patient Positioning: Place the patient in the lithotomy position on the examination table.
- Insertion of Speculum: Gently insert the speculum into the vagina to visualize the cervix.
- Sample Collection:
- Ectocervical cells: Use Ayre’s spatula to scrape cells from the squamous epithelium of the ectocervix.
- Endocervical cells: Use a cytobrush to collect cells from the columnar epithelium within the endocervical canal.
- Using a broom-like device, rotate 360° to collect cells from the ectocervix and endocervix.
- Smear Preparation:
- For conventional smears: Spread the sample evenly on a glass slide and immediately fix it with spray fixative.
- For liquid-based cytology (LBC): Place the sampling device in the liquid preservative vial and swirl it to release cells.
- Labeling: Label the slide or vial with the patient’s details.
- Transportation: Send the sample to the laboratory for analysis.
Fixative
Proper fixation is crucial to preserve cellular morphology and prevent air-drying artifacts.
- Conventional Cytology Fixative:
- 95% ethanol or spray fixatives containing polyethylene glycol.
- Fixation should be done immediately after smearing the sample to prevent drying.
- Liquid-Based Cytology Fixative:
- Prepackaged vials containing preservatives like SurePath or PreservCyt.
- The fixative preserves the cellular components and removes obscuring elements like blood and mucus.
Staining Procedure
The Papanicolaou (Pap) stain is the standard staining method for cervical cytology due to its ability to provide excellent cytoplasmic and nuclear detail.
Steps in Pap Staining
- Fixation: Fix the smear using 95% ethanol or liquid preservative.
- Hydration: Gradually rehydrate the slide in descending alcohol concentrations (e.g., 95%, 70%).
- Nuclear Staining:
- Use Hematoxylin to stain the nuclei blue.
- Cytoplasmic Staining:
- Orange G-6 (OG-6): Stains keratinized cells orange.
- EA-36 or EA-50 (Eosin Azure): Differentiates non-keratinized squamous cells, staining them pink, green, or blue depending on maturity.
- Dehydration: Pass the slide through increasing alcohol concentrations (70%, 95%, 100%).
- Clearing: Use xylene to make the slide transparent.
- Mounting: Apply a coverslip with a mounting medium.
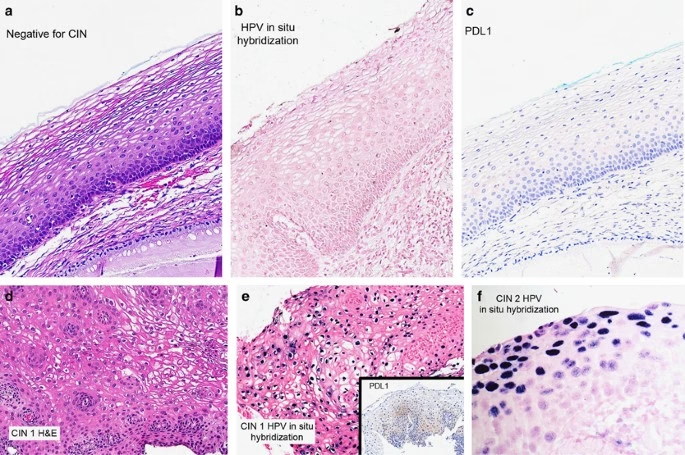
Detection of Malignant and Premalignant Lesions
Premalignant Lesions
Premalignant changes are classified based on The Bethesda System (TBS):
- ASC-US: Atypical squamous cells of undetermined significance.
- ASC-H: Atypical squamous cells cannot exclude HSIL.
- LSIL: Low-grade squamous intraepithelial lesion (indicative of HPV-related changes).
- HSIL: High-grade squamous intraepithelial lesion (moderate to severe dysplasia).
Malignant Lesions
Malignant changes typically include:
- Squamous Cell Carcinoma:
- Irregular, hyperchromatic nuclei.
- Coarse chromatin and increased nuclear-to-cytoplasmic ratio.
- Adenocarcinoma:
- Enlarged glandular cells with hyperchromatic nuclei and prominent nucleoli.
- Multinucleation or vacuolation of cytoplasm may be present.
Features of Cytological Findings
| Lesion Type | Nuclear Features | Cytoplasmic Features | Example Conditions |
| Normal Cells | Uniform nuclei, smooth chromatin | Cytoplasm proportionate | Healthy cervix |
| LSIL | Mild nuclear enlargement, slight hyperchromasia | Cytoplasmic perinuclear halo | HPV-related changes |
| HSIL | Marked nuclear enlargement, hyperchromasia | Cytoplasm reduced | CIN2, CIN3 |
| Squamous Cell Carcinoma | Irregular nuclear membranes, coarse chromatin | Dense, keratinized cytoplasm | Invasive cervical cancer |
| Adenocarcinoma | Enlarged, pleomorphic nuclei | Vacuolated or mucinous cytoplasm | Glandular carcinoma |
Clinical Application and Screening Guidelines
- Cervical cytology is most effective when combined with HPV testing, especially in women over 30 years of age.
- Screening Recommendations:
- Women aged 21–29 years: Pap smear every 3 years.
- Women aged 30–65 years: Pap smear every 3 years or co-testing with HPV every 5 years.