Collection of Cytologic Specimens
Purpose and Importance:
- The accuracy of cytological diagnoses heavily relies on collecting adequate, representative samples from the targeted site.
- Proper collection ensures cellular morphology is preserved and sufficient cells are present for evaluation.
- For the female genital tract, this means obtaining cells from the cervix, particularly the transformation zone, where pre-cancerous and cancerous changes are most likely to occur.
Techniques for Sample Collection:
- Exfoliative Cytology:
- This technique collects naturally shed or gently scraped cells from mucosal or epithelial surfaces. The method varies slightly based on the site within the female genital tract (e.g., cervix, vagina).
- The preferred technique for the cervix is using a spatula or cervical brush to scrape cells from the ectocervix (outer cervix) gently and the transformation zone (squamocolumnar junction). This ensures that cells from the areas most susceptible to dysplasia are collected.
- Instrumentation:
- Ayer’s Spatula: Used to scrape cells from the ectocervix and is effective for obtaining samples from the transformation zone.
- Endocervical Brush: A small, soft-bristled brush used to collect cells from the endocervical canal. This is particularly important for younger women and patients with deep transformation zones where cells are not easily accessible with a spatula alone.
- Broom-Type Devices: Some modern practices use broom-like devices to simultaneously sample both the ectocervix and endocervix simultaneously, reducing patient discomfort and improving cell collection efficiency.
- Handling Techniques:
- Cells should be spread thinly and evenly on a glass slide immediately after collection to prevent clumping and air-drying artifacts.
- Rapid fixation is essential to preserve cellular integrity and avoid artifacts that can interfere with accurate diagnosis.
Preparation Techniques: –
Conventional Smear: The sample is spread directly onto a slide. While quick and cost-effective, it can lead to cell clumping or overlapping.
Liquid-Based Cytology (LBC): Cells are rinsed into a preservative fluid rather than spread on a slide. LBC allows for improved cellular preservation, removal of obscuring materials like blood and mucus, and a more uniform cell distribution on the slide.
Processing of Cytologic Specimens
Purpose of Processing: Proper processing ensures the cells are preserved, arranged appropriately on the slide, and prepared for accurate staining. This involves steps like fixation and slide preparation, especially in liquid-based cytology.
-
Fixation:
- Importance: Fixation is crucial as it prevents autolysis (self-digestion) and decomposition of cells, preserving nuclear and cytoplasmic details. Immediate fixation also prevents air-drying, which can create artifacts, especially in Pap smears.
- Common Fixatives:
- Alcohol-Based Fixatives: 95% ethanol is a standard fixative in Pap smears. The sample is either immersed in ethanol or sprayed with an alcohol-based fixative directly on the slide.
- Alternative Fixatives: Methanol and isopropanol are sometimes used, but 95% ethanol remains the most widely accepted for its compatibility with Pap staining.
- Spray Fixation: Often alcohol-based, it is convenient when immediate immersion in ethanol is impractical.
- Air-Drying: This technique is avoided in Pap smears due to the risk of artifacts. However, air-dried samples are preferred in specific cases (e.g., Diff-Quik staining).
-
Slide Preparation:
- Direct Smears: The sample is smeared onto the slide in conventional cytology. This method is quick and convenient but has drawbacks, such as cell overlapping and uneven distribution.
- Liquid-Based Cytology (LBC): Cells are collected in a preservative fluid and then processed to remove debris and achieve a monolayer of cells. In the lab, cells are filtered and transferred onto a slide in a controlled manner, providing cleaner slides with uniform cell distribution.
Staining of Cytologic Specimens
Staining is essential for visualizing cell morphology, allowing the differentiation of normal and abnormal cells. The choice of stain depends on the type of cytologic examination and the cellular details required.
Purpose of Staining: Staining enhances contrast by highlighting nuclear and cytoplasmic structures, making it easier to identify cellular abnormalities indicative of infections, dysplasia, or malignancy.
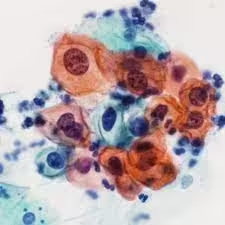
Papanicolaou (Pap) Stain:
Introduction
- The Pap is a cytological stain developed to identify and assess cellular abnormalities, especially in cervical smears.
- Its effectiveness lies in its ability to highlight cellular and nuclear details and provide contrast between different cell types.
- The Pap smear is primarily used in cervical cancer screening programs but is also applicable to other body sites.
- Its sensitivity and specificity in detecting dysplastic and malignant changes make it invaluable for early detection and diagnosis, aiding in reducing the incidence and mortality of cervical cancer.
Principle of Pap Stain
- The principle of the Pap stain is to differentiate cells based on the varying affinities of cellular components for different dyes.
- This differential staining helps pathologists distinguish between cell types, their maturity, and any dysplastic or malignant changes.
- The Pap stain uses a sequence of acidic and basic dyes that react with specific cellular components:
- Hematoxylin: Stains the nucleus blue by binding to chromatin, allowing for detailed examination of nuclear size, shape, and chromatin pattern.
- Orange G (OG-6): A cytoplasmic stain that selectively stains keratinized cells orange, assisting in identifying mature squamous cells.
- Eosin Azure (EA): A mixture of eosin and light green dyes that impart various colors to different cell types based on their cytoplasmic components. Eosin stains the cytoplasm of mature cells pink, while light green stains immature cells blue to green.
Reagents Used in Pap Stain
- Hematoxylin: Stains the nuclei blue; Harris or Gill’s hematoxylin is commonly used.
- OG-6 (Orange G): An orange cytoplasmic stain used to differentiate keratinized cells, typically in a 0.5% or 1% concentration.
- EA Solution (Eosin Azure): A blend of eosin, light green, and bismarck brown dyes in alcohol. There are different formulations of EA (EA-36, EA-50, and EA-65), varying slightly in their dye concentrations.
- Ethanol: Used for both dehydration and fixation steps.
- Acetic Acid: Enhances nuclear staining by adjusting the pH during hematoxylin staining.
- Distilled Water: Used for rinsing slides during the staining procedure.
Procedure of Pap Stain
- Fixation: Immediately fix the smear with 95% ethanol or an alcohol-based spray fixative to preserve cellular details.
- Hematoxylin Staining:
- Place the fixed slide in Harris or Gill’s hematoxylin for 3-5 minutes to stain the nuclei blue.
- Rinse in running tap water to remove excess stains.
- Differentiate in 0.5% hydrochloric acid-alcohol for a few seconds (if necessary) to enhance nuclear contrast.
- Rinse again in water and a mild alkaline solution (e.g., lithium carbonate or tap water) to develop a clear blue nuclear stain.
- Orange G (OG-6) Staining:
- Immerse the slide in OG-6 solution for 2-3 minutes. This step stains keratinized cells orange.
- Rinse in 95% ethanol to remove excess stain.
- Eosin Azure (EA) Staining:
- Transfer the slide to EA solution for 3-5 minutes. This solution stains the cytoplasm of mature cells pink and immature or abnormal cells in shades of blue or green.
- Rinse in 95% ethanol to remove excess EA stain.
- Dehydration and Clearing:
- Sequentially immerse the slide in increasing ethanol concentrations (95% to 100%) to dehydrate.
- Clear in xylene or another clearing agent, ensuring all water and alcohol are removed from the tissue sections.
- Mounting:
- Apply a mounting medium and coverslip the slide to protect it and facilitate microscopic examination.
Results and Interpretation of Pap Stain
- Nuclear Staining: Nuclei appear blue to dark blue. The nuclear details (size, shape, chromatin pattern) are crucial in identifying cellular abnormalities such as increased nuclear size, hyperchromasia, and irregular nuclear contours, all indicative of dysplasia or malignancy.
- Cytoplasmic Staining:
- Keratinized Cells: These cells stain bright orange, indicative of fully mature squamous cells.
- Superficial Squamous Cells: Cytoplasm appears pink or eosinophilic due to eosin in the EA solution.
- Parabasal and Intermediate Cells: These cells may stain shades of blue to green due to the light green component of EA, which highlights less mature cells.
- Pathological Findings:
- Infectious Agents: Organisms like Candida, Trichomonas vaginalis, and Herpes simplex virus can sometimes be visualized, aiding diagnosis.
- Dysplastic and Malignant Cells: Features include a high nuclear-to-cytoplasmic ratio, irregular nuclear contours, hyperchromasia, and coarse chromatin. These changes are hallmarks of dysplasia and carcinoma.
Alternative Stains:
-
- Romanowsky Stains (e.g., Diff-Quik, Giemsa): Used in FNAC and non-gynecological cytology, these stains are quick and effective for identifying microorganisms and assessing cell morphology, especially for hematologic or inflammatory cells.
- Hematoxylin and Eosin (H&E) Stain: Often used in histology, H&E highlights general cellular structures but lacks the specificity of Pap staining.
- Special Stains:
-
-
- Acid-Fast Stain (e.g., Ziehl-Neelsen): Highlights acid-fast organisms like Mycobacterium tuberculosis, useful in sputum cytology.
- Periodic Acid-Schiff (PAS) Stain: Stains carbohydrates in fungi, mucin, and certain tumor cells, aiding in identifying infections or specific tumors.
- Immunocytochemistry: Techniques that use antibodies to stain specific proteins, such as p16 and Ki-67 in cervical cytology, are increasingly used to detect cellular changes associated with high-risk HPV infections.
-
Quality Control and Troubleshooting
Quality Control:
- High-quality sample preparation and staining are essential to avoid diagnostic errors. Cytology labs have protocols for ensuring specimen quality, which include reviewing sample adequacy, fixing errors, and standardizing staining procedures.
- Common quality checks ensure cells are well-preserved, evenly distributed, and free of overlapping or debris. This includes avoiding artifacts due to air-drying, overstaining, or contamination.
Troubleshooting Common Issues:
- Air-Drying Artifacts: When slides are not immediately fixed, cells can shrink or swell, losing nuclear detail and causing chromatin to appear smudged or unclear. Immediate fixation with an alcohol-based fixative prevents this.
- Uneven Staining: This may result from inconsistent application of reagents or contamination. Ensuring fresh reagents, clean slides, and proper technique can prevent this issue.
- Background Debris: Blood, mucus, or inflammatory cells can obscure the view. In LBC, debris is removed before cells are deposited on the slide, minimizing this problem.